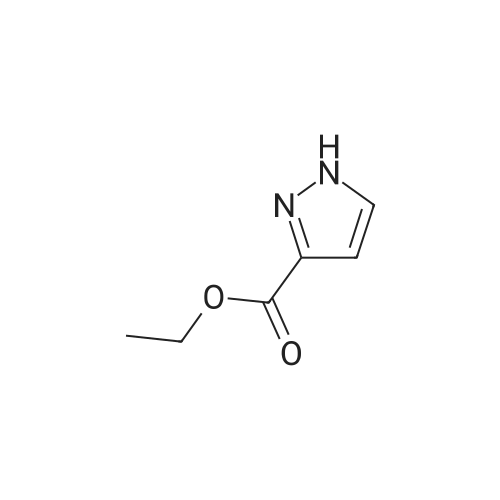
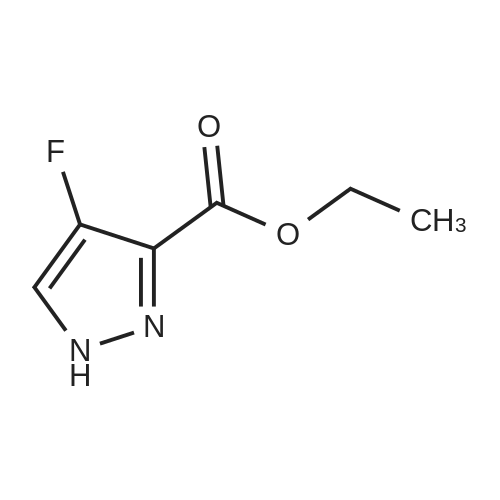
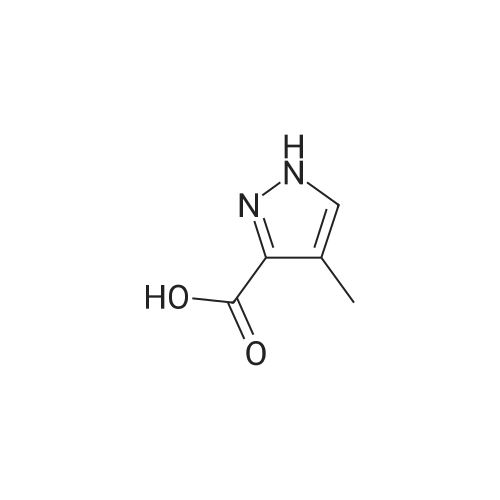
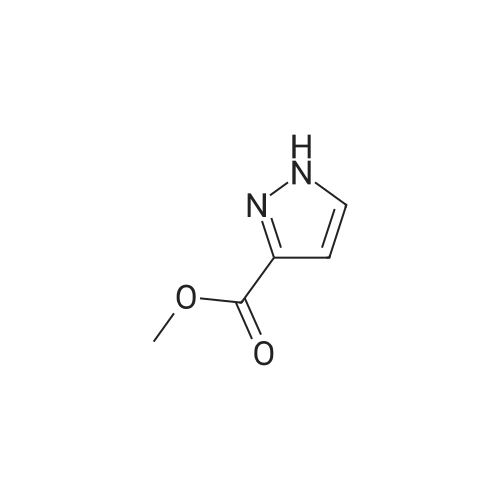
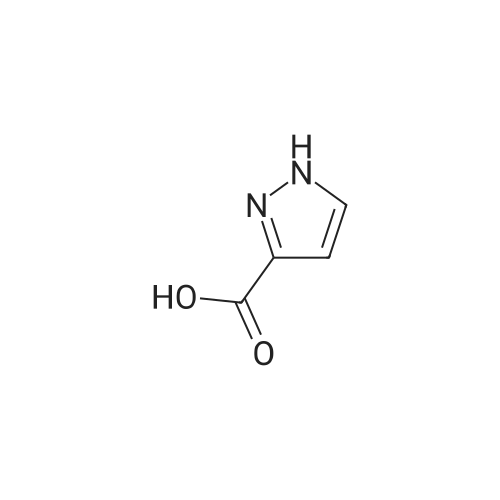
| 80% | With sulfuric acid;Heating / reflux; | 1 H-Pyrazole-3-carboxylic acid (2.0 g, 17.84 mmol) was dissolved in ethanol (20 ml_), sulfonic acid (1 ml_) was added and the reaction mixture was heated to reflux overnight. Evaporation in vacuo followed by addition of water and aqueous sodium carbonate gave a white precipitate. Filtration followed by wash with water gave 1 H- pyrazole-3-carboxylic acid ethyl ester (2.0 g, 80percent) as white crystals. |
| 72% | With sulfuric acid; for 24h;Reflux; | General procedure: To a suspension of 4-methoxy-3,5-dimethylbenzoic acid (4 g, 22.2 mmol) in MeOH (60 mL) sulphuric acid was added (1 mL) and mixture stirred at reflux for 1 day. Water was added and it was extracted with AcOEt, organic layers were put together and dried over sodium sulphate and concentrated. The oil thus obtained was purified by normal phase chromatography with 5percent MeOH/DCM to yield the title compound as a colorless oil (99percent yield). LRMS: m/z 195 (M+1)+ Retention time: 6.17 min (Method B) 1H NMR (250 MHz, DMSO-d6) delta ppm 2.30 (s, 6 H) 3.74 (s, 3 H) 3.87 (s, 3 H) 7.71 (s,2H).; Obtained (72percent) from 1H-pyrazole-3-carboxylic acid following the procedure described in Preparation 117, using ethanol as solvent. LRMS: m/z 139 (M-1)+ Retention time: 2.13 min (Method A) |
| 72% | With sulfuric acid; for 12h;Reflux; | Example 14A1 lH-Pyrazole-3-carboxylic acid ethyl ester To a solution of lH-pyrazole-3-carboxylic acid (2.0 g, 17.8 mmol) in anhydrous ethanol (20 mL) was added concentrated H2SO4 (1 mL) at room temperature. The mixture was refluxed for 12 hours. TLC (neat EtOAc) indicated that the reaction was completed. The mixture was concentrated, the residue was washed with aqueous NaHC03 (20 mL), extracted with dichloromethane (50 mL x 3). The organic layer was dried over Na2S04, filtered, and concentrated in vacuum to give Example 14A1 (1.8 g, yield 72percent) as a white solid. |
| | Description 1; Ethyl 1H-pyrazole-3-carboxylate (D1); 1 H-pyrazole-3-carboxylic acid (1.Og, 8.9 mmol), was dissolved in ethanol (~12ml) and sulphuric acid added (1.5ml). the solution was heated at reflux overnight. The reaction mixture was concentrated in vacuo and water (-2OmI) added, the solution was neutralised with sodium bicarbonate. Ethylacetate (~70ml) was added and the layers partitioned, the aq was extracted with ethylacetate (~70ml) and the combined organics were dried (MgSO4) and concentrated in vacuo to yield the title compound as a white solid (1.21g, 8.64 mmol). deltaH (CDCI3, 400 MHz): 7.77 (1 H, d), 6.86 (1 H, d),4.43 (2H, quart), 1.42 (3H, t). |
| With sulfuric acid; for 20h;Heating / reflux; | 1 - ( [2-(trimethylsilyl)ethoxy]methyl) - lH-pyrazole-3 -carbaldehyde was prepared as follows: 3-Methyl pyrazole (50 g, 0.61 mol) was placed in a 5 L round-bottom flask equipped with mechanical stirrer. 3 L of water was added and heated to 80 C. KMn04 (211.90 g, 1.34 mol) was added portion wise and refluxed for 4.5 h. After stirring at rt overnight, solid was filtered and washed with water. The water was removed in vacuo and 100 mL of water was kept in the flask which was acidified with 1 N HCl to pH 4. It was extracted with EtOAc (2x 1L), washed with brine (2x150 mL), dried over MgS04, filtered and removed in vacuo to yield 1H-pyrazole-3-carboxylic acid (38 g, 56percent) as a white solid. 38 g (0.34 mol) of IH-pyrazole-3-carboxylic acid was refluxed in anhydrous ethanol (1 L) and conc. sulfuric acid (60 mL) for 20 h under nitrogen. Ethanol was removed and crude was basified to pH 8. Precipitated solid was filtered. The filtrate was extracted with THF/CHC13 (2: 3, 3x 1 L), dried over MgS04, filtered and removed in vacuo to yield ethyl 1H-pyrazole-3- carboxylate (39 g, 82percent) as a white solid. To a suspension of ethyl 1H-pyrazole-3-carboxylate (4.42 g, 31.57 mmol) in 1,4-dioxane (140 mL) under N2 atmosphere at 0 C was added NaH (0.91 g, 37.88 mmol) and stirred for 15 min. Neat SEM-Cl (5.79 g, 34.73 mmol) was added drop wise to reaction mixture and stirred overnight at rt. It was quenched with water (30 mL) and excess 1,4-dioxane was removed in vacuo. The residue was extracted with EtOAc (2x250 mL), washed with water (1x50 mL), dried over MgS04, filtered and removed in vacuo to give crude ethyl 1 - [2- (trimethylsilyl)ethoxy]methyl}-1H pyrazole-3-carboxylate (8.84 g) as a yellow oil. The crude material was used in next step without purification. To a suspension of LiAlH4 in THF (100 mL) at 0 C under N2 atmosphere was added a solution of ethyl 1- f [2-(trimethylsilyl)ethoxy]methyl}-1H pyrazole-3-carboxylate (8.88 g, 32.88 mmol) slowly. After addition was completed the cooling bath was removed and reaction mixture was stirred overnight. It was quenched with water (10 mL) carefully at 0 C. THF was removed and residue was diluted with DCM (250 mL) and organic layer was separated, dried over MgS04 and removed in vacuo. The crude material was plugged thru a pad of silica gel with EtOAc/hexanes (from 10percent to 100percent) to yield (1-[2- (trimethylsilyl)ethoxy]methyl) -lH-pyrazol-3-yl)methanol (5.80 g, 77percent) as an yellow oil. 53.75 g (0.24 mol) of (1- f [2-(trirnethylsilyl)ethoxy]methyl}-1H pyrazol-3-yl)methanol was dissolved in THF and 122.97 g (1.41 mol) of Mn02 was added. The resulting mixture was refluxed for 60 h. Solid material was filtered through a pad of celite and washed with hot THF. The filtrate was removed in vacuo to give crude product. The crude was plugged thru a pad of silica gel and eluted with EtOAc/hexanes (from 20percent to 50percent) to yield 1-[2- (trimethylsilyl)ethoxy]methyl}-1H pyrazole-3-carbaldehyde (50.88 g, 86.5percent) as a red oil. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping