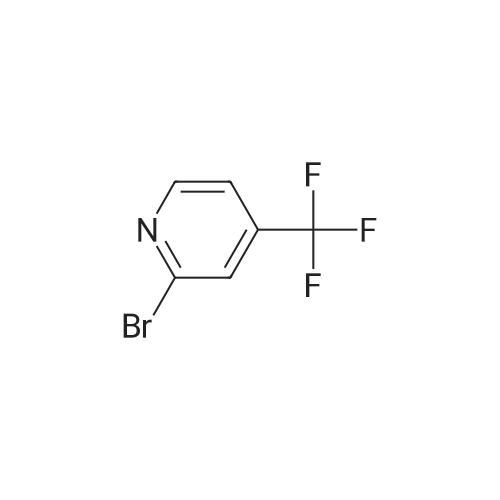
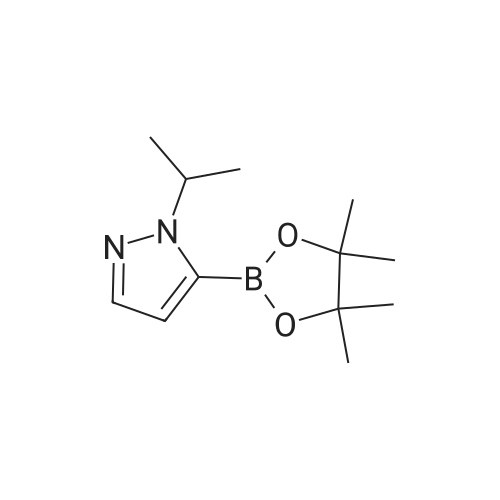
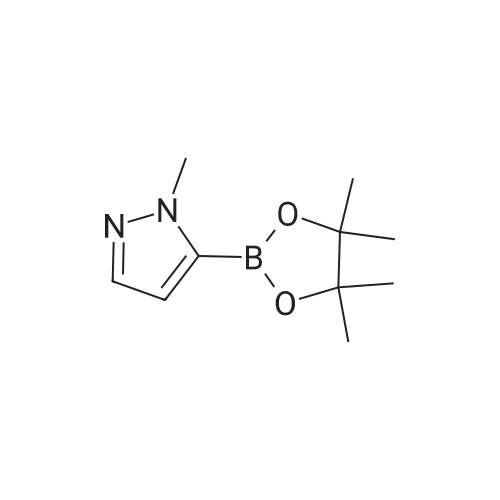
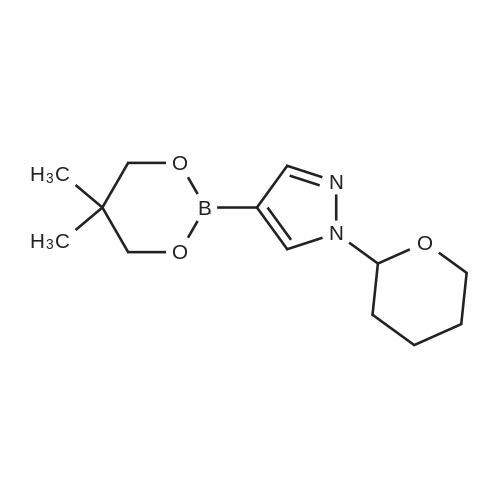
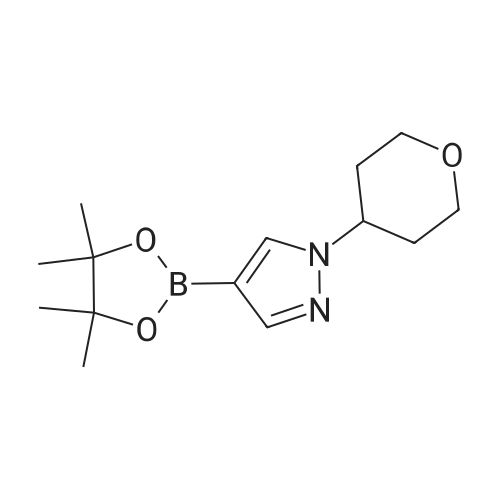
| 89% | With tetrakis(triphenylphosphine) palladium(0); potassium carbonate; In ethanol; toluene; at 100℃; for 2h; | <strong>[175205-81-9]2-bromo-4-(trifluoromethyl)pyridine</strong> (2.0 g, 8.85 mmol) was added to a mixture of 1-(tetrahydro-2H-pyran-2-yl)-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole (2.85 g,9.73 mmol,) tetrakis(triphenylphosphine)palladium(0) (511 mg, 0.44 mmol,) and potassium carbonate (3.67 g 26.55 mmol,) in ethanol (10 mL) and toluene (10 mL). The mixture was stirred at 100°C for 2h. The reaction was cooled down and water was added. The aqueous phase was extracted with 3X50 mL of dichloromethane. The organic phase was dried with MgSO4, filtered and concentrated. Purification by flash chromatography (100percent heptane to 100percent ethyl acetategradient, 40 g column) afforded 2.34 g (89percent) of 2-(2-tetrahydropyran-2-ylpyrazol-3-yl)-4-(trifluoromethyl)pyridine as a yellow oil. LCMS (Method Shimadzu): RT = 1.45 min, m+H = 298.1. 1H NMR (400 MHz, Chloroform-d) delta 8.87 (d, J = 5.0 Hz, I H), 7.86 (d, J = 1.5 Hz, 1H), 7.63 (dd, J = 14.6,2.2Hz, 1H), 7.48 (dd, J = 5.2, 1.6 Hz, 1H), 6.70 (d, J = 1.9 Hz, 1H), 6.14 (dd, J = 10.0,2.5Hz, 1H),4.04(ddt,J= 11.5,4.3,2.1 Hz, 1H),3.61 (td,J= 11.4,2.6Hz, 1H),2.63-2.48(m, 1H),2.17-1.99 (m, 2H), 1.83 - 1.62 (m, 2H), 1.58 (s, 1H). |
| With tetrakis(triphenylphosphine) palladium(0); sodium carbonate; In ethanol; water; toluene; at 90℃; for 2h; | General procedure: Pd(PPh3)4 (9.23 g, 7.99 mmol) and a 2 mol/L aqueous Na2CO3solution (2.4 L) were added to an EtOH (1.6 L)/ toluene (1.6 L)mixed solution of 1 (500 g, 1.8 mol) and 2-bromo-5-fluoropyridine(281.2 g, 1.60 mol), and the mixture was heated to 90 C and stirredfor 2 h. After standing to cool at 0 C, water was added to thereaction mixture, followed by extraction with EtOAc. The extractedorganic layer was distilled off under reduced pressure. Theobtained residue was stirred in EtOAc (1.2 L), and NH silica gelwas added thereto. The mixture was stirred at room temperaturefor 1 h. Then, the silica gel was filtered off, and the solvent was distilledoff under reduced pressure to obtain 5-fluoro-2-(1-(tetrahydro-2H-pyran-2-yl)-1H-pyrazol-5-yl)pyridine as a brown oil.A 4 mol/L HCl?EtOAc solution (1.2 L) was added to a solution of5-fluoro-2-(1-(tetrahydro-2H-pyran-2-yl)-1H-pyrazol-5-yl)pyridinein MeOH (1.0 L), and the mixture was stirred at room temperaturefor 5 h. The deposited solid was then collected by filtration. Theobtained solid was stirred in water (2.0 L), and an 8 mol/L aqueousNaOH solution (0.3 L) was added thereto under ice cooling. Themixture was extracted with EtOAc, and the solvent was distilledoff under reduced pressure. The obtained residue was stirred for1 h in Et2O. Then, the deposited solid was collected by filtrationand dried by heating under reduced pressure to obtain the titlecompound 2a as a colorless powder (185 g, 71percent over 2 steps). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping