Alternatived Products of [ 58457-98-0 ] Product Details of [ 58457-98-0 ]
| CAS No. : | 58457-98-0 | MDL No. : | MFCD03701203 |
| Formula : |
C17H24N2O6 | Boiling Point : | - |
| Linear Structure Formula : | - | InChI Key : | MDMZRMHNXPKKND-ZDUSSCGKSA-N |
| M.W : |
352.38
| Pubchem ID : | 10831821 |
| Synonyms : | |
Application In Synthesis of [ 58457-98-0 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 58457-98-0 ]
- 1
 [ 186581-53-3 ]
[ 186581-53-3 ]
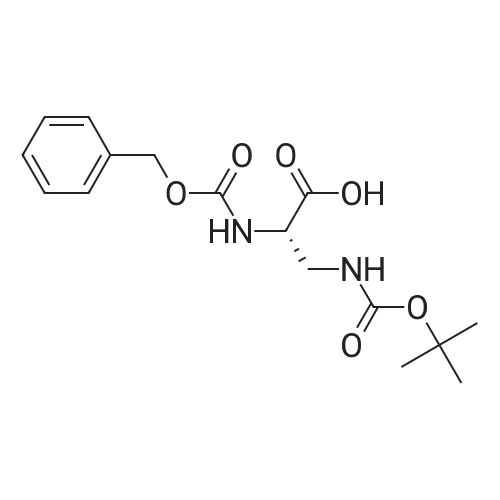 [ 16947-84-5 ]
[ 16947-84-5 ]
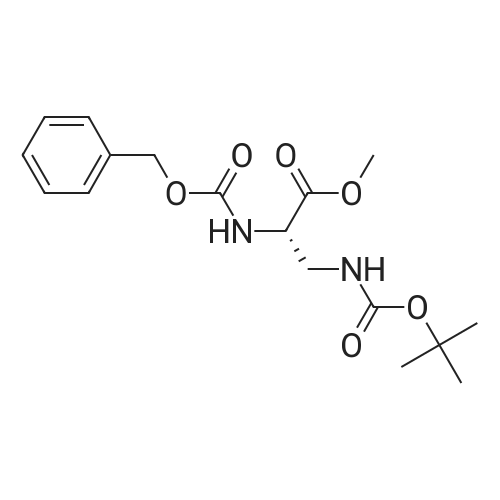 [ 58457-98-0 ]
[ 58457-98-0 ]
Reference: [1]Journal of Medicinal Chemistry,1998,vol. 41,p. 2786 - 2805 [2]Journal of Medicinal Chemistry,1981,vol. 24,p. 567 - 572 [3]Journal of Medicinal Chemistry,1976,vol. 19,p. 766 - 772 [4]Journal of the American Chemical Society,1992,vol. 114,p. 998 - 1010 - 2
 [ 58457-98-0 ]
[ 58457-98-0 ]
 [ 16947-84-5 ]
[ 16947-84-5 ]
| Yield | Reaction Conditions | Operation in experiment |
|---|
| | Methylene chloride (150 ml) and a saturated aqueous solution (250 ml) of sodium bicarbonate were added to methyl (2S)-2-amino-3-[(tert-butoxycarbonyl)amino]propanoate hydrochloride (15.0 g) to separate the layers. The aqueous layer thus obtained was extracted with methylene chloride (2 .x. 150 ml). The organic layers were combined and dried over anhydrous sodium sulfate. The solvent was distilled off under reduced pressure. A pale yellow oil thus obtained and pyridine (13.6 ml) were dissolved in acetonitrile (250 ml). Under ice cooling, an acetonitrile (25 ml) solution of benzyl choroformate (8.78 ml) was added dropwise to the resulting solution over 10 minutes. After stirring at room temperature for 5 hours, the reaction mixture was concentrated under reduced pressure. Ethyl acetate (250 ml) and a 10percent aqueous solution (200 ml) of citric acid were added to the residue thus obtained to separate the layers. The organic layer was washed with saturated saline (200 ml), a saturated aqueous solution (200 ml) of sodium bicarbonate and saturated saline (200 ml) and dried over anhydrous magnesium sulfate. The solvent was distilled off under reduced pressure. A colorless oil thus obtained was solidified with hexane and then washed with hexane to yield a crudely purified product (17.8 g) of methyl (2S)-2-[(benzyloxy)carbonyl]amino}-3-[(tert-butoxycarbonyl)amino]propanoate. The ester (17.8 g) thus obtained was dissolved in a mixed solvent of tetrahydrofuran (200 ml) and water (40 ml). Lithium hydroxide (1.34 g) was added and the mixture was stirred at room temperature for 1.5 hours. Under reduced pressure, the solvent was distilled off. To the residue thus obtained were added ethyl acetate (200 ml) and a 10percent aqueous solution (200 ml) of citric acid to separate the layers. The organic layer was dried over anhydrous magnesium sulfate. Hexane (250 ml) was added to the residue obtained by distilling off the solvent under reduced pressure. The precipitate thus obtained was collected by filtration, whereby the title compound (16.9 g) was obtained.1H-NMR(DMSO-d6)delta: 1.36(9H,s), 3.19-3.31(2H,m), 4.02-4.11(1H, m), 5.01(1H, d, J=12. 7Hz), 5.05(1H, d, J=12. 7Hz), 6.83 (1H, t, J=5.7Hz), 7.28-7.45(6H,m), 12. 67 (1H,br s). MS (ESI)m/z: 361((M+Na)+]. |
- 3
 [ 16947-84-5 ]
[ 16947-84-5 ]
 [ 74-88-4 ]
[ 74-88-4 ]
 [ 58457-98-0 ]
[ 58457-98-0 ]
| Yield | Reaction Conditions | Operation in experiment |
|---|
| With caesium carbonate; In N,N-dimethyl-formamide; at 0 - 20℃; for 3.58333h; | Preparation of Compound BBA solution of (S)-2-Benzyloxycarbonylamino-3-tert-butoxycarbonylamino-propionic acid (Compound AA) (30.0 g, 88.8 mmol) in DMF (100 mL) was cooled down to 0° C., followed by the addition of CsCO3 (28.9 g, 88.8 mmol). The reaction was stirred for 5 min, followed by the dropwise addition of MeI (6.6 mL, 106.6 mmol). The reaction was allowed to rise to ambient temperature, and then was stirred for 1 h. Additional amounts of MeI (6.6 mL, 106.6 mmol, each) were then added after 30 min and 60 min respectively. The reaction mixture was then stirred for 1 h at ambient temperature. Solvents were removed in vacuo, and the residue was dissolved in EtOAc (800 mL), and washed with water (3.x.300 mL) and brine (300 mL). The organic layer was separated and dried over MgSO4. The solvent was removed in vacuo to afford crude Compound BB in 94percent yield (29.6 g, 83.8 mmol) as an amorphous solid. LC-MS [M+H] 353.0 (C17H24N2O6+H, calc: 353.4). Compound BB was used directly in the next reaction without further purification. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science




















