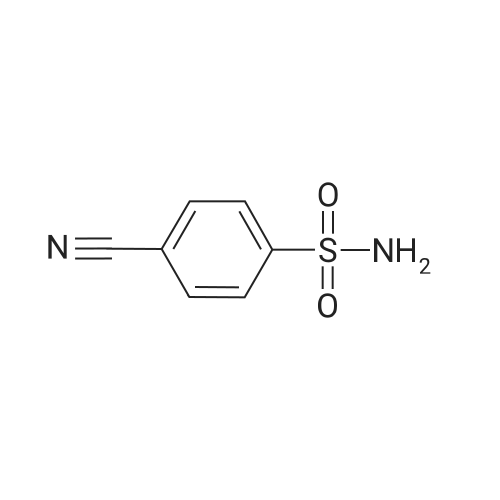
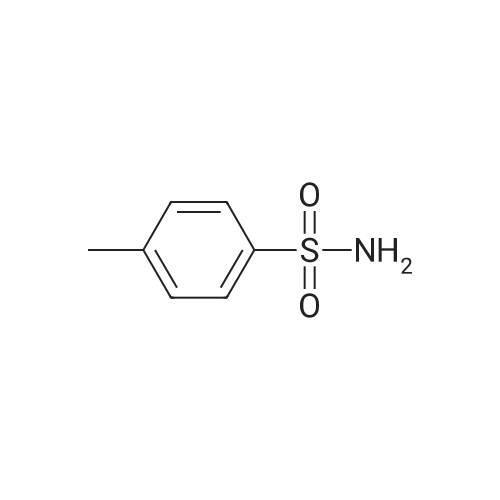
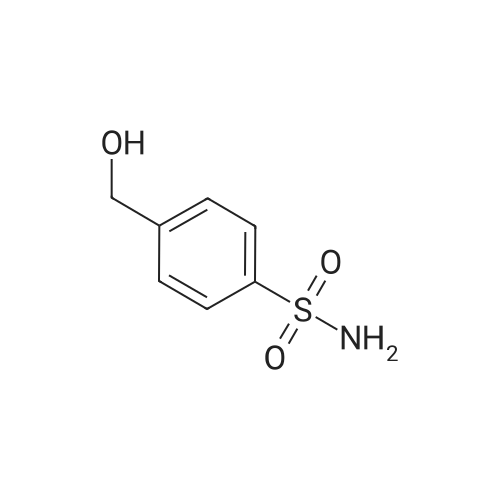
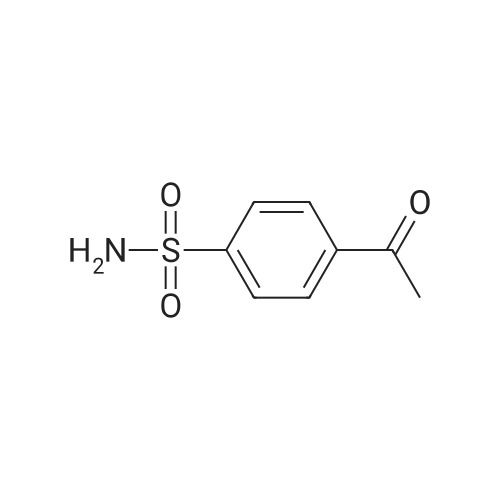
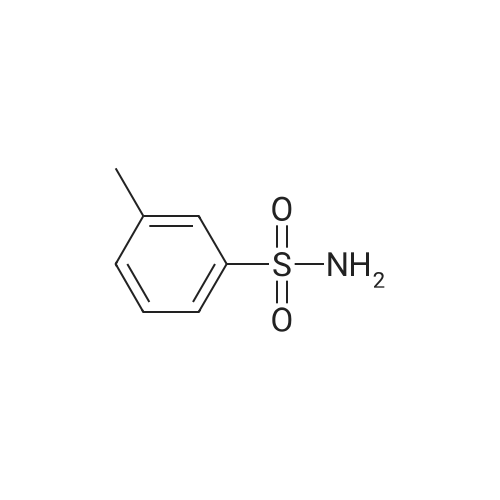
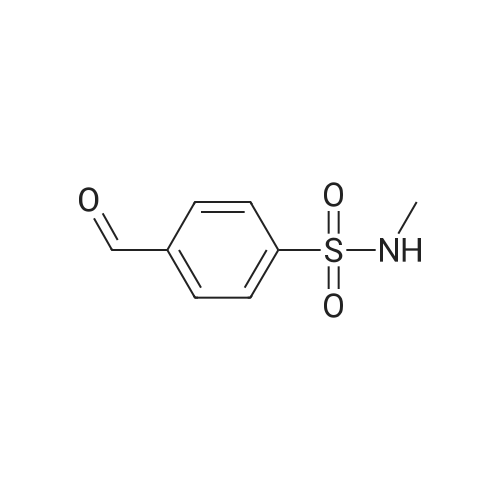
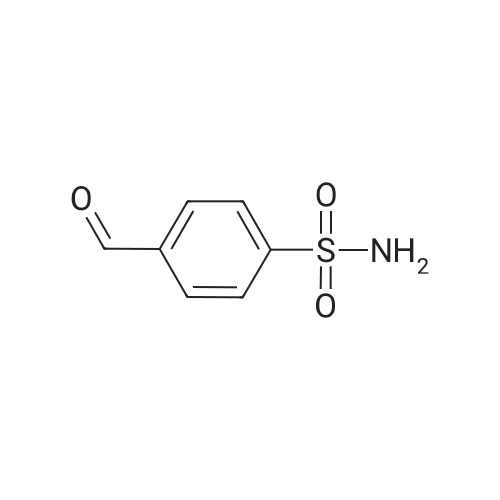
| 12% | With sulfuric acid; In ethanol; water; for 2h;Reflux; | EXAMPLE 5. Preparation of 13. Concentrated sulfuric acid (7 mL) was added dropwise to the mixture of acetic anhydride (80 niL) and acetic acid (40 mL). The mixture was cooled with ice bath. 4- Methylbenzenesulfonamide 1 1 (12 g, 70 mmol) was added and the reaction temperature was maintained beneath 5 C. Chromium oxide (8g, 80 mmol) was added in batches. Then the reaction mixture was stirred for 4 h at 5-10 C, then the solution was poured into ice water (500 mL). The aqueous solution was extracted with DCM (3* 100 mL). The combined organic phase was washed with brine, dried over anhydrous sodium sulphate and concentrated to give (4-sulfamoylphenyl)methylene diacetate 12 as a yellow oil. The yellow oil which was achieved in the last step was dissolved in ethanol (10 mL). Water ( 10 mL) and concentrated sulfuric acid (2 mL) was added. The reaction mixture was heated to reflux and stirred for 2 h. Solvent was concentrated and the residue was diluted with water (50 mL). The aqueous solution was extracted with ethyl acetate (2*20 mL). The combined organic phase was dried over sodium sulphate and concentrated. The residue was purified by column on silica gel (eluent: Hexane/EA 3: 1 to 1 : 1 ) to afford 13 as a white solid (1.5 g, 12% yield). FontWeight="Bold" FontSize="10" H NMR (400 MHz, DMSO) δ 10.09 (s, 1 H), 8.10 (d, J - 8.3 Hz, 2H), 8.03 (d, J - 8.3 Hz, 2H), 7.59 (s, 2H). |
| | Example I; Preparation of the sodium salt of N-methoxycarbonyl-4-(4,5-dihydro-l-phenyl-3-trifluoromethyl-lH-pyrazole-S-yl)benzenesulphonamide (Compound 31 of Table 1); (a) and (b) 4-Formil benzenesulphonamide; AcO OAcCrO3/H2SO4-----------»•(a)H2S04 /EtOH(b)*"o=s=oo=s=oo=s=oNH,Acetic anhydride (400 ml) is placed in a 1000 ml flask, cooled to -10C, CrO3 (70g, 0.7 moles) is added and stirred for In at 0C.In another flask p-toluenesulphonamide (40g, 234 mmoles) is suspended in acetic anhydride (350 ml) , cooled to -10C and H2SO<i (60 ml) is added dropwise and slowly, since the reaction is quite exothermic. The solid is dissolved entirely. It is left for 15 min at 0C and the solution of the oxidising mixture, prepared independently as indicated above, is added to it. Once the addition is finished it is stirred for 2.5 h at 0C. It is then poured onto ice-water (approximate ratio of 7:1, 4 litres) and it is stirred overnight. The resulting solution is extracted with AcOEt, the organic solution is washed with water, 'dried over sodium sulphate, filtered and evaporated to dryness.To the resulting pasty mixture, ethanol (130 ml), water (40 ml) and H2S04 (8 ml) are added, and it is heated at reflux for 2.5 h. It is cooled, solvent is eliminated in a rotavapor and the residue is separated between AcOEt and water. The organic phase is washed with a 5% potassium carbonate solution and water. Once the organic solution is dried and evaporated, there remain 13.4 g (31%) of crude, cream-coloured solid, which is used without purifying in the next stage.IR (KBr, cm'1) : 3357, 3244, 1701, 1337, 1172:H NMR (de-DMSO, 5 ppm) : 7.57 (s, 2H) , 8.0 (d,J=8.2Hz, 2H), 8.1 (d, J=8.2Hz, 2H) , 10.1 (s, IH) |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping