Alternatived Products of [ 20349-89-7 ] Product Details of [ 20349-89-7 ]
| CAS No. : | 20349-89-7 | MDL No. : | MFCD00067757 |
| Formula : |
C10H12O3 | Boiling Point : | - |
| Linear Structure Formula : | - | InChI Key : | - |
| M.W : |
180.20
| Pubchem ID : | - |
| Synonyms : | |
Application In Synthesis of [ 20349-89-7 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 20349-89-7 ]
- 1
 [ 67-56-1 ]
[ 67-56-1 ]
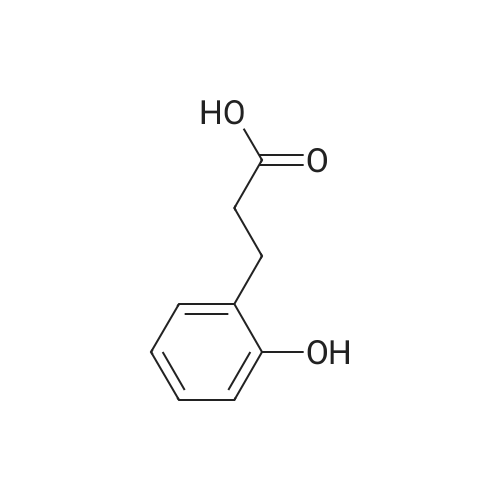 [ 495-78-3 ]
[ 495-78-3 ]
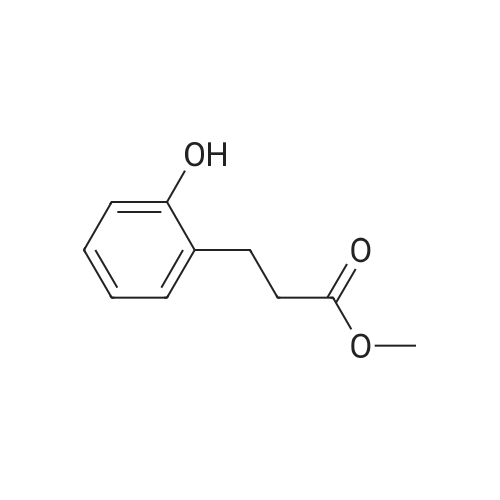 [ 20349-89-7 ]
[ 20349-89-7 ]
- 2
 [ 186581-53-3 ]
[ 186581-53-3 ]
 [ 495-78-3 ]
[ 495-78-3 ]
 [ 20349-89-7 ]
[ 20349-89-7 ]
| Yield | Reaction Conditions | Operation in experiment |
|---|
| 25 mg | In acetone; | Precultured G. cingulata (5 mL) was transferred into a 500 mL Erlenmeyer flask containing 300 mL of medium. Cultivation was carried out at 27 C with stirring (ca. 120 rpm) for 3 days. After the growth of G. cingulata, 50 mg of 1 in 1.0 mL of dimethyl sulfoxide (DMSO) was added into the medium and cultivated for an additional 7 days, together with two controls, which contained either mycelia with medium or substrate dissolved in DMSO with medium. No metabolic product was observed in two controls. After the fermentation, the culture medium and mycelia were separated by filtration. The medium was saturated with NaCl and extracted with EtOAc. The mycelia were also extracted with EtOAc. Each EtOAc extract was combined, the solvent was evaporated, and a crude extract (423 mg) was obtained. The extract was distributed between 5% NaHCO3 aq and EtOAc, and EtOAc phase was evaporated to give a neutral fraction (159 mg). No metabolite was detected by TLC and HPLC. The alkali phase was acidified to pH 3 with 1 N HCl and distributed between water and EtOAc. The EtOAc phase was evaporated, and the acidic fraction (264 mg) was obtained. Metabolites were detected from both fractions by TLC and HPLC, respectively. The acidic fraction was dissolved in acetone (5 mL), and CH2N2 (1 mL) was added to the fraction. The solution was evaporated, and the methylation fraction was obtained. The methylation fraction was subjected to silica-gel column chromatography (CC) (silica gel 60, 230-400 mesh, Merck) with a n-hexane-Et2O gradient (9:1 to 1:4) to yield compound 2a (25 mg). Compound 2a (13 mg) was dissolved in MeOH (1 mL), 1% NaOH (2 mL) added to the solution, and the solution was refluxed for 30 min. The solution was acidified to pH 3 with 1 N HCl and distributed between EtOAc and water. The EtOAc phase was evaporated to give 2 (9 mg, Rt=6.6 min). |
- 3
 [ 67-56-1 ]
[ 67-56-1 ]
 [ 495-78-3 ]
[ 495-78-3 ]
- 3-(2-hydroxy-5-nitrophenyl)propanoic acid methyl ester [ No CAS ]

- 3-(2-hydroxy-3-nitrophenyl)propanoic acid methyl ester [ No CAS ]

 [ 20349-89-7 ]
[ 20349-89-7 ]
- 4
 [ 20349-89-7 ]
[ 20349-89-7 ]
 [ 495-78-3 ]
[ 495-78-3 ]
| Yield | Reaction Conditions | Operation in experiment |
|---|
| 9 mg | | Precultured G. cingulata (5 mL) was transferred into a 500 mL Erlenmeyer flask containing 300 mL of medium. Cultivation was carried out at 27 C with stirring (ca. 120 rpm) for 3 days. After the growth of G. cingulata, 50 mg of 1 in 1.0 mL of dimethyl sulfoxide (DMSO) was added into the medium and cultivated for an additional 7 days, together with two controls, which contained either mycelia with medium or substrate dissolved in DMSO with medium. No metabolic product was observed in two controls. After the fermentation, the culture medium and mycelia were separated by filtration. The medium was saturated with NaCl and extracted with EtOAc. The mycelia were also extracted with EtOAc. Each EtOAc extract was combined, the solvent was evaporated, and a crude extract (423 mg) was obtained. The extract was distributed between 5% NaHCO3 aq and EtOAc, and EtOAc phase was evaporated to give a neutral fraction (159 mg). No metabolite was detected by TLC and HPLC. The alkali phase was acidified to pH 3 with 1 N HCl and distributed between water and EtOAc. The EtOAc phase was evaporated, and the acidic fraction (264 mg) was obtained. Metabolites were detected from both fractions by TLC and HPLC, respectively. The acidic fraction was dissolved in acetone (5 mL), and CH2N2 (1 mL) was added to the fraction. The solution was evaporated, and the methylation fraction was obtained. The methylation fraction was subjected to silica-gel column chromatography (CC) (silica gel 60, 230-400 mesh, Merck) with a n-hexane-Et2O gradient (9:1 to 1:4) to yield compound 2a (25 mg). Compound 2a (13 mg) was dissolved in MeOH (1 mL), 1% NaOH (2 mL) added to the solution, and the solution was refluxed for 30 min. The solution was acidified to pH 3 with 1 N HCl and distributed between EtOAc and water. The EtOAc phase was evaporated to give 2 (9 mg, Rt=6.6 min). |
- 5
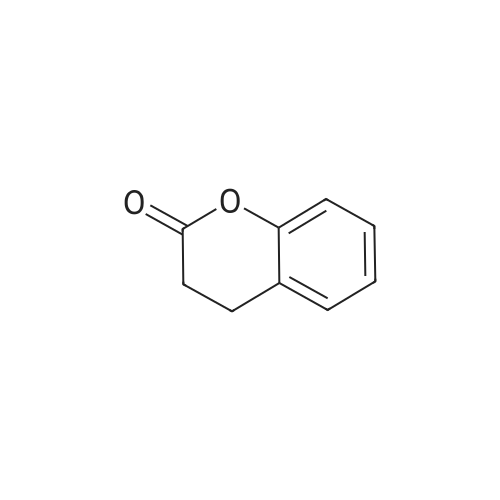 [ 119-84-6 ]
[ 119-84-6 ]
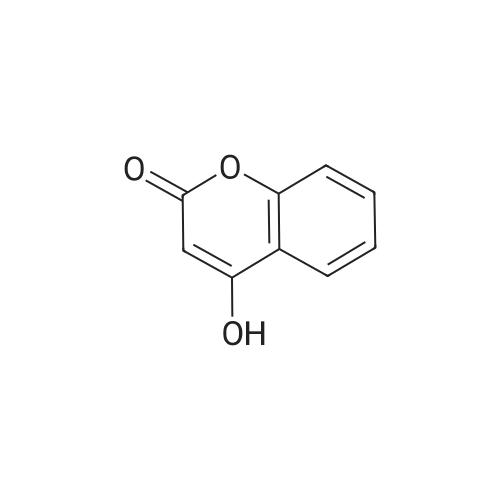 [ 1076-38-6 ]
[ 1076-38-6 ]
 [ 2669-94-5 ]
[ 2669-94-5 ]
 [ 495-78-3 ]
[ 495-78-3 ]
 [ 1318928-52-7 ]
[ 1318928-52-7 ]
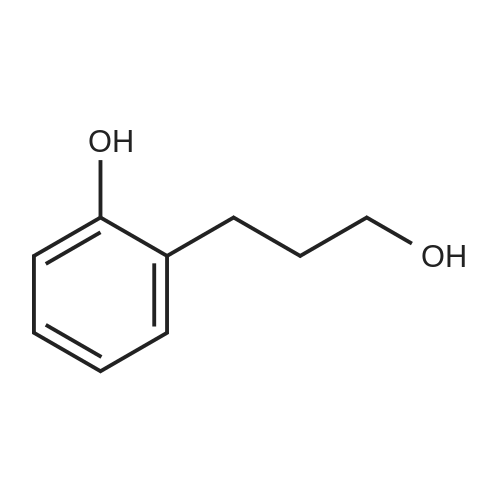 [ 1481-92-1 ]
[ 1481-92-1 ]
 [ 20349-89-7 ]
[ 20349-89-7 ]
- 6
 [ 495-78-3 ]
[ 495-78-3 ]
 [ 74-88-4 ]
[ 74-88-4 ]
 [ 20349-89-7 ]
[ 20349-89-7 ]
- 7
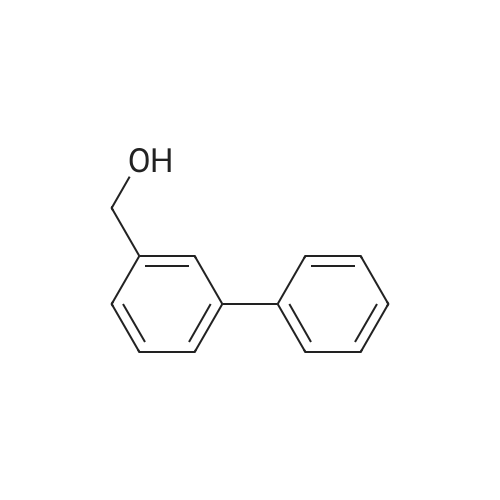 [ 69605-90-9 ]
[ 69605-90-9 ]
 [ 20349-89-7 ]
[ 20349-89-7 ]
- C23H22O3 [ No CAS ]

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science

























