Alternatived Products of [ 143157-27-1 ] Product Details of [ 143157-27-1 ]
| CAS No. : | 143157-27-1 | MDL No. : | |
| Formula : |
C23H18F2N2O7 | Boiling Point : | - |
| Linear Structure Formula : | - | InChI Key : | - |
| M.W : |
472.40
| Pubchem ID : | - |
| Synonyms : | |
Safety of [ 143157-27-1 ]
| Signal Word: | | Class: | |
| Precautionary Statements: | | UN#: | |
| Hazard Statements: | | Packing Group: | |
Application In Synthesis of [ 143157-27-1 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 143157-27-1 ]
- 1
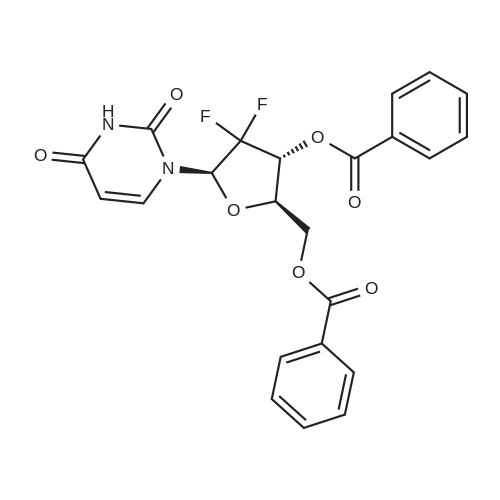 [ 143157-27-1 ]
[ 143157-27-1 ]
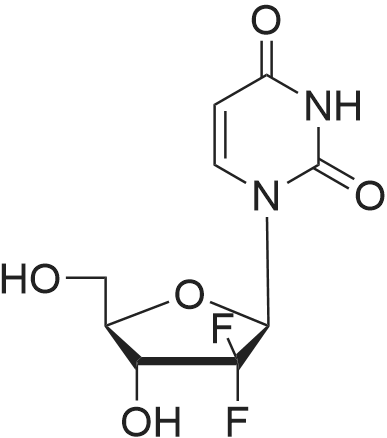 [ 114248-23-6 ]
[ 114248-23-6 ]
| Yield | Reaction Conditions | Operation in experiment |
|---|
| 99% | With ammonia; In methanol; at 20℃; for 12h; | Compound 68-3 was dissolved in NH3/MeOH (600 mL) and stirred overnight. The solvent was concentratedto give the residue, which was purified by silica gel column chromatography (5% MeOH in DCM) to give68-4 (12 g, 99%) as a white solid |
| 99% | With ammonia; In methanol; | Compound 68-3 was dissolved in NH3/MeOH (600 mL) and stirred overnight. The solvent was concentrated to give the residue, which was purified by silica gel column chromatography (5% MeOH in DCM) to give 68-4 (12 g, 99%) as a white solid. |
| 95.8% | With ammonia; In methanol; at 20℃; for 15h; | P24-3 (39.2 g, 83 mmol) was dissolved in saturated methanolic ammonia, and the resulting solution was stirred at R.T. for 15 hours. The solvent was removed, and the residue was purified on a silica gel column (DCM/MeOH=50:1 to 20:1) to give P24-4 (21.0 g, 95.8%). |
| 95.8% | With ammonia; In methanol; at 20℃; for 15h; | j0298j Compound P24-3 (39.2 g, 83 mmol) was dissolved in saturated methanolic ammonia, and the resulting solution was stirred at R.T. for 15 hours. The solvent was removed, and the residue was purified on a silica gel column (DCM/MeOH = 50:1 to 20:1) to give P24-4 (21.0 g, 95.8%). |
| 12 g | With ammonia; In methanol; | Compound 68-3 was dissolved in NH3/MeOH (600mL) and stirred overnight. The solvent was concentrated togive the residue, which was purified by silica gel columnchromatography (5% MeOH in DCM) to give 68-4 (12 g,99%) as a white solid. |

Reference: [1]Patent: US2015/105341,2015,A1 .Location in patent: Paragraph 0676; 1030; 1311 [2]Patent: US2015/366887,2015,A1 .Location in patent: Paragraph 0543; 0546 [3]Patent: US2013/165400,2013,A1 .Location in patent: Paragraph 0420; 0421 [4]Patent: WO2014/209979,2014,A1 .Location in patent: Paragraph 0298 [5]Journal of Medicinal Chemistry,2015,vol. 58,p. 1862 - 1878 [6]Patent: US2015/366888,2015,A1 .Location in patent: Paragraph 0594; 0597 - 2
 [ 98-88-4 ]
[ 98-88-4 ]
 [ 114248-23-6 ]
[ 114248-23-6 ]
 [ 143157-27-1 ]
[ 143157-27-1 ]
| Yield | Reaction Conditions | Operation in experiment |
|---|
| 72% | With 4-methyl-morpholine; dmap; In tetrahydrofuran; at 0 - 5℃; for 8h; | formula 7(0.61 g, 2.31 mmol, 1 eq.), 4-dimethylaminopyridine (0.14 g, 0.5 eq.), 4-Methylmorpholine (1.78 ml, 7 eq.) Was dissolved in tetrahydrofuran (12 ml) Followed by cooling. And benzoyl chloride (0.67 ml, 2.5 eq.) Was added slowly, Followed by stirring at 5 DEG C or lower for 8 hours. Thin layer chromatography afforded the compound of formula (7) After confirming whether or not the residue remained, it was concentrated under reduced pressure. A saturated aqueous sodium hydrogen carbonate solution (10 ml) and After diluting with brine (7 ml) and methylene chloride (30 ml), only the organic layer was separated. The separated organic layer was dried over anhydrous sodium sulfate and concentrated under reduced pressure. After concentration under reduced pressure, the residue was separated by column chromatography (ethyl acetate: hexane = 1: 1) Compound (8a) (0.79 g) which was a white solid was obtained in a yield of 72%. |
Categories

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping







