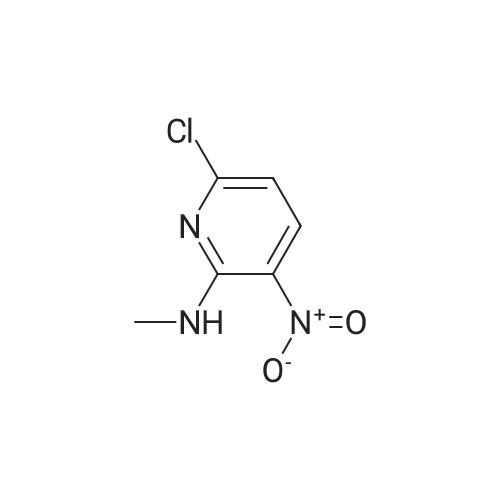
| 59.7% | With iron; ammonium chloride; In methanol; water; at 20℃; for 3.0h;Reflux; | To a solution of <strong>[33742-70-0]6-chloro-N-methyl-3-nitropyridin-2-amine</strong> (630 mg, 3.36 mmol) in MeOH (10 ml) and water (10 ml) was added Fe (940.8 mg, 16.8 mmol) and NH4C1 (898.8 mg, 168 mmol) at 20C. The mixture was stirred for 3 hours under reflux temperature until TLC (PE: EA =3: 1) showed that the reaction was complete. The mixture was filtered, the filtrate concentrated and residue treated with water (10 ml) and extracted with ethyl acetate (2x20 ml). The combined organic layers were washed with brine (30 ml), dried over Na2S04 and concentrated to give the desired product (316 mg, 59.7%) as a yellow solid which was used in next step without further purification. LCMS (m/z): 158.1 [M+H]+. |
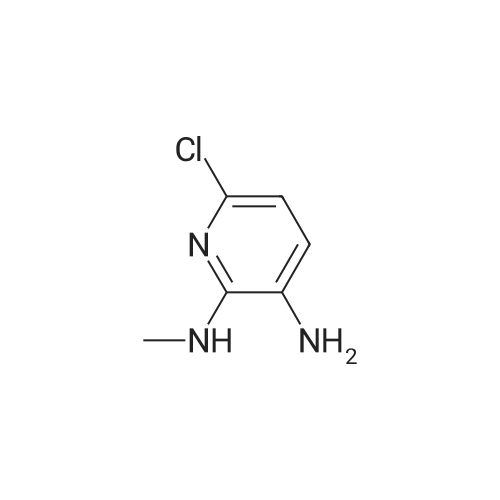
| With hydrogenchloride; stannous chloride dihydrate; In water;Reflux; | 6-Chloro-N-methyl-3-nitropyridin-2-amine (28-1, 10.5 g, 56 mmol) and tin(II) chloride dihydrate (50.5 g, 224 mmol) were suspended in concentrated HCl (80 mL) and refluxed overnight. The solution was cooled to room temperature and then added very slowly to a NaOH/ethyl acetate solution at -78 C, until the solution had a slightly basic pH. The suspension was washed with sodium bicarbonate, brine, dried over sodium sulfate, filtered, and concentrated to produce 6-chloro-N2-methylpyridine-2,3-diamine (28-2) as a black solid. HRMS (M+H)+: observed =158.0471, calculated =158.0480. |
| | 6-chloro-N2-methylpYridine-2,3-diamine (1-2) <strong>[33742-70-0]6-chloro-N-methyl-3-nitropyridin-2-amine</strong> (l-l)(10.5g, 56 mmol) and Tin(II) chloride dihydrate(50 g, 220 mmol) were suspended in concentrated HC1 (80 mL) and refluxed overnight. The solution was cooled to room temperature and then added very slowly to a NaOH/Ethyl acetate solution at -78C, until the solution had a slightly basic pH. The suspension was washed with sodium bicarbonate, brine, dried over sodium sulfate, filtered, and concentrated to produce the black solid 6-chloro-N2-methylpyridine-2,3-diamine(l~2). MS (M+H)+: observed = 158.0, calculated = 158.6. |
| | 6-Chloro-N-methyl-3-nitropyridin-2-amine (1-1, 10.5 g, 56 mmol) and tin(II) chloride dehydrate (50.5 g, 224 mmol) were suspended in concentrated HC1 (80 mL) and refiuxed overnight. The solution was cooled to room temperature and then added very slowly to a NaOH/ethyl acetate solution at -78 C, until the solution had a slightly basic pH. The suspension was washed with sodium bicarbonate, brine, dried over sodium sulfate, filtered, and concentrated to produce 6-chloro-N2-methylpyridine-2,3-diamine (1-2) as a black solid. |
| With hydrogenchloride; tin(II) chloride dihdyrate; In water;Reflux; | 6-Chloro-N2-methylpyridine-2,3-diamine (1-2)6-Chloro-N-methyl-3-nitropyridin-2-amine (1-1, 10.5 g, 56 mmol) and tin(II) chloride dehydrate (50.5 g, 224 mmol) were suspended in concentrated HCI (80 mL) and refluxed overnight. The solution was cooled to room temperature and then added very slowly to a NaOH/ethyl acetate solution at -78C, until the solution had a slightly basic pH. The suspension was washed with sodium bicarbonate, brine, dried over sodium sulfate, filtered, and concentrated to produce 6-chloro-N2-methylpyridine-2,3-diamine (1-2) as a black solid. HRMS (M+H)+: observed = 158.0487, calculated = 158.0480. |
| With hydrogen; In tetrahydrofuran; methanol; at 20℃; for 23.0h; | Step 102.2: 6-chloro-N2-methylpyridine-2,3-diamine The title compound was prepared in analogy to the procedure described in Step 85.2 using <strong>[33742-70-0]6-chloro-N-methyl-3-nitropyridin-2-amine</strong> (Step 102.1) at RT for 23 hr. The crude material was purified by silica gel column chromatography (25% EtOAc/hexane) to afford a purple solid. tR: 0.64 min (LC-MS 2); ESI-MS: 158 [M+H]+ (LC-MS 2); Rf=0.12 (25% EtOAc/Hexane). |
| With hydrogen; In tetrahydrofuran; at 20℃; for 23.0h; | To a solution of 5-bromo-N,3-dimethyl-2-nitroaniline (Step 85.1) (2.7 g, 11.02 mmol) in THF(100 mL) and MeOH (100 mL) was added Raney Nickel (189 mg, 2.203 mmol) and the resultingmixture was stirred under hydrogen atmosphere at RT for 16 hr. The reaction was filtered through a pad of Celite and the resulting filtrate was concentrated under reduced pressure to afford the title product (2.5 g, 10.56 mmol, 96% yield) as off-white solid. tR: 0.94 mm (LC-MS 2); ESl-MS: 214 [M+H] (LC-MS 2). The title compound was prepared in analogy to the procedure described in Step 85.2 using <strong>[33742-70-0]6-chloro-N-methyl-3-nitropyridin-2-amine</strong> (Step 102.1) at RT for 23 hr. The crude material waspurified by silica gel column chromatography (25% EtOAc/hexane) to afford a purple solid. tR:0.64 mm (LC-MS 2); ESl-MS: 158 [M+H] (LC-MS 2); R = 0.12 (25% EtOAc/Hexane). |
| With iron; acetic acid; In ethanol; water; | 3.0 g <strong>[33742-70-0]6-chloro-N-methyl-3-nitro-pyridin-2-amine</strong> (16 mmol) was dissolved in the mixture of 30 ml ethanol and 15 ml water then 4.47 g iron powder (80 mmol, 5 eq.) was added. To this mixture 1.2 ml glacial acetic acid was added dropwise then the reaction mixture was refluxed until no further conversion was observed. The reaction mixture was filtered, the filtrate was concentrated under reduced pressure and the residue was purified via flash chromatography using DCM as eluent to give 6-chloro-2-methylamino-3-aminopyridine. MS(M+H) = 158.2 |
| With iron; acetic acid; at 80℃; for 3.0h; | To a mixture of <strong>[33742-70-0]6-chloro-N-methyl-3-nitropyridin-2-amine</strong> (15.8 g, 84.2 mmol) in AcOH (100 mL) was added iron powder (15.4 g, 276 mmol). The yellow mixture was stirred at 80 C. for 3 h. The reaction was cooled to RT and filtered. The filtercake was washed with EtOAc (2*100). The combined organic layers were concentrated under reduced pressure and the crude product was purified by flash chromatography (120 g silica gel, 50% EtOAc/PE) to afford 3-amino-6-chloro-2-methylaminopyridine (8.40 g, 63% yield) as a brown solid. 1H NMR (CDCl3) delta 6.80 (d, 1H), 6.50 (d, 1H), 3.39 (br s, 2H), 3.01 (s, 3H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science