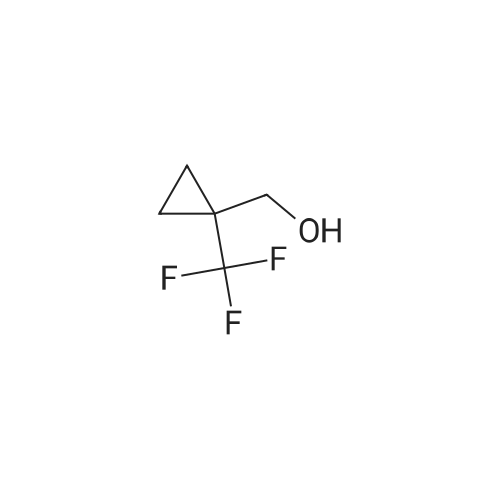
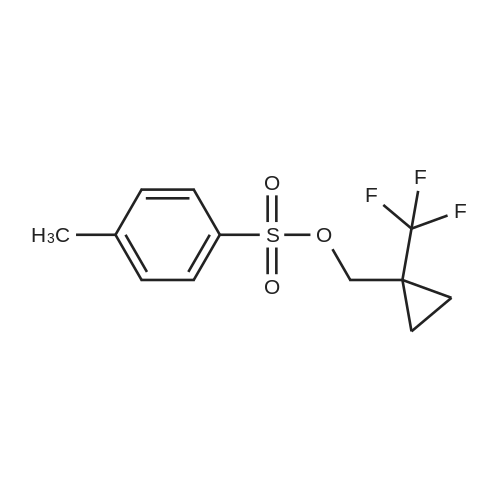
| 70% | With dmap; triethylamine; In dichloromethane; at 20℃; for 12h; | [01043]A mixture of [1-(trifluoromethyl)cyclopropyl]methanol (2.6 g, 18.56 mmol, 1.00 equiv), dichloromethane (30 mL), TEA (5.63 g, 55.64 mmol, 3.00 equiv), 4-dimethylaminopyridine (2271.86 mmol, 0.10 equiv), and 4-methylbenzene-1-sulfonyl chloride (4.26 g, 22.36 mmol, 1.20 equiv) was stirred for 12 h at room temperature. The resulting solution was diluted with ethyl acetate, washed with brine, dried over anhydrous sodium sulfate, and concentrated under vacuum. The residue was purified by a silica gel colunm eluting with ethyl acetate/petroleum ether to afford the title compound (3.8 g, 70%) as colorless oil. GCMS [mlz] 294. |
| 70% | With triethylamine; In 4-(dicyanomethylene)-2-methyl-6-(4-dimethylaminostyryl)-4H-pyran; at 20℃; for 2h;Cooling with ice; | To a solution of <strong>[371917-17-8](1-(trifluoromethyl)cyclopropyl)methanol</strong> (350 mg, 2.5 mmol) and TEA (757.5 mg, 7.5 mmol) in DCM (5 mL) in an ice-water bath, was added TsCl (573 mg, 3.0 mmol) in portions. The mixture was allowed to warm to room temperature and stirred for 2 h. The solvent was removed under reduced pressure. The residue was purified by prep-HPLC to give the product as a colorless oil (0.52 g, yield: 70%). |
| 66% | With dmap; triethylamine; In dichloromethane; at 0 - 20℃; | A 0 C. solution of <strong>[371917-17-8](1-(trifluoromethyl)cyclopropyl)methanol</strong> (2.46 g, 17.56 mmol), TEA (2.94 mL, 21.1 mmol) and DMAP (0.215 g, 1.76 mmol) in DCM (35 mL) was treated with p-toluenesulfonyl chloride (3.38 g, 17.7 mmol), allowed to warm to RT and stirred overnight. The mixture was treated with additional DCM, washed with 2N HCl (3×), followed by satd. NaHCO3, dried over Na2SO4, concentrated to dryness and purified via silica gel chromatography (EtOAc/Hex) to afford (1-(trifluoromethyl)cyclopropyl)methyl 4-methylbenzenesulfonate (3.42 g, 66%). 1H NMR (400 MHz, DMSO-d6): δ 7.77 (m, 2H), 7.48 (m, 2H), 4.16 (s, 2H), 2.41 (s, 3H), 1.04 (m, 2H), 0.92 (m, 2H); MS (ESI) m/z: 295.1 (M+H+). |
| 49% | With dmap; triethylamine; In dichloromethane; at 0 - 20℃; | Step 2: p-Toluenesulfonyl chloride (9.6 g, 50.35 mmol) was added at 0 C. to a mixture of (1-trifluoromethyl-cyclopropyl)-methanol (7 g, 49.96 mmol), triethylamine (7.7 mL, 55.24 mmol) and DMAP (0.61 g, 4.996 mmol) in 100 mL of DCM. The resulting mixture was stirred at 0 C. to RT overnight before being washed with aqueous 1M HCl. The aqueous layer was back extracted twice with DCM. The combined organic layers were dried (Na2SO4), filtered, and evaporated. The residue was purified by SiO2 flash chromatography (120 g SiO2, hexanes/[hexanes/Et2O 8/2] 100 to 0% hexanes) to give 7.26 g of toluene-4-sulfonic acid 1-trifluoromethyl-cyclopropylmethyl ester (49% yield). |
| With dmap; triethylamine; In dichloromethane; at 0 - 20℃; | Preparation 52; [1- (trifluoromethyl) cyclopropyl] methyl 4-methylbenzenesulfonate; To a solution of 1- (trifluoromethyl) cyclopropyl] methanol (J. Fluorine Chem. , 2001,109, 2,95, 8.18 g, 58.4 mmol) in dichloromethane (50 ml), at 0C, was added triethylamine (50 ml), 4-dimethylaminopyridine (713 mg, 5.84 mmol) and p-toluenesulphonyl chloride (11.1 g, 58.4 mmol). The reaction mixture was allowed to warm to room temperature and stirred overnight. The reaction mixture was concentrated in vacuo and the residue was partitioned between diethyl ether (250ml) and hydrochloric acid (0. 5M, 100 ml). The two layers were separated and the aqueous phase was extracted with diethyl ether (100 ml). The combined organic phases were washed with saturated aqueous sodium hydrogencarbonate solution (50 ml) and brine (50 ml), dried (MgS04) and concentrated in vacuo. The residue was purified using a BiotageT" Flash 40 system with gradient elution, diethyl ether: cyclohexane [5: 95 to 20: 80]. The appropriate fractions were combined and concentrated to give the titled compound (11.8 g). 'H-NMR (CDCI3) : 0.81-0. 89 (2H), 1.09-1. 16 (2H), 2.44-2. 48 (3H), 4.09-4. 12 (2H), 7.33-7. 39 (2H), 7.77-7. 82 (2H) |
| With pyridine; In dichloromethane; at 20 - 50℃; for 5h; | The residue was dissolved in dichloromethane (4ml), pyridine (0.48ml, 6mmol) and p- toluenesulfonyl chloride (1.1g, 6mmol) were added. The mixture was stirred for 3 hours at room temperature, warmed at 50C for 2 hours, cooled to room temperature, then evaporated. Water was added then 2M hydrochloric acid and the mixture was extracted with diethyl ether (x3). combined organic extracts were dried (MgS04) and evaporated to dryness to give 100mg of the title compound. 1HNMR CDCI3 δ: 0.82-0.86(2H,m), 1.10-1.13(2H,m), 2.46 (3H.s), 4.09 (2H,s), 7.35 (2H,d), 7.78 (2H,d). |
| With dmap; In dichloromethane; at 25℃; for 18h; | To a round-bottom flask were added (1- (trifluoromethyl) cyclopropyl) methanol (4200 mg, 30.0 mmol) , DCM (120 mL) , 4-methylbenzene-1-sulfonyl chloride (7430 mg, 39.0 mmol) , N, N-dimethylpyridin-4-amine (366 mg, 3.00 mmol) and N, N-dimethylpyridin-4-amine (366 mg, 3.00 mmol) . The reaction mixture was stirred for 18 h at 25 . The mixture was concentrated in vacuum and the residue was purified by normal phase chromatography (ISCO, SiO2, 40 g Agela Flash column, 0-5EA/PE, 40 min, dry loaded) to give the title compound.1H NMR (CDCl3, 400 MHz) : δ 7.77 (d, J 8.22 Hz, 2H) , 7.47 (d, J 7.83 Hz, 2H) , 4.16 (s, 2H) , 2.40 (s, 3H) , 1.03 (s, 2H) , 0.92 (bs, 2H) . |
| 7.3 g | With dmap; triethylamine; In dichloromethane; at 0 - 20℃; for 15h;Inert atmosphere; | To a stirred solution of <strong>[371917-17-8](1-(trifluoromethyl)cyclopropyl)methanol</strong> (5.11 g, 38.96 mmol) in anhydrous dichloromethane (80 mL) was added triethylamine (16.3 mL, 116.9 mmol) at 0 C under argon atmosphere, followed by 4-methylbenzenesulfonyl chloride (9.62 g, 50.6 mmol) and 4-dimethylaminopyridine (436 mg, 3.9 mmol). The reaction mixture was stirred at room temperature for 15 h. The reaction mixture was diluted with dichloromethane (80 mL), and organic layer was washed with 2 M HCl (90 mL), saturated aqueous sodium bicarbonate solution (80 mL), and brine (80 mL). The organic solution was dried over anhydrous sodium sulfate, filtered and concentrated to give (1-(trifluoromethyl)cyclopropyl)methyl 4-methylbenzenesulfonate as a light yellow oil (7.30 g, 64% over two steps). LCMS: LC retention time 2.08 min. MS (ESI) m/z 295 [M+H]+. 1H NMR (400 MHz, chloroform-d) δ 7.79 (d, J = 8.0 Hz, 2H), 7.36 (d, J = 8.0 Hz, 2H), 4.10 (s, 2H), 2.46 (s, 3H), 1.12 (m, 2H), 0.84 (m, 2H) ppm. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping