Alternatived Products of [ 85293-46-5 ] Product Details of [ 85293-46-5 ]
| CAS No. : | 85293-46-5 | MDL No. : | MFCD13195450 |
| Formula : |
C10H18O4 | Boiling Point : | No data available |
| Linear Structure Formula : | - | InChI Key : | - |
| M.W : |
202.25
| Pubchem ID : | - |
| Synonyms : | |
Application In Synthesis of [ 85293-46-5 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 85293-46-5 ]
- 1
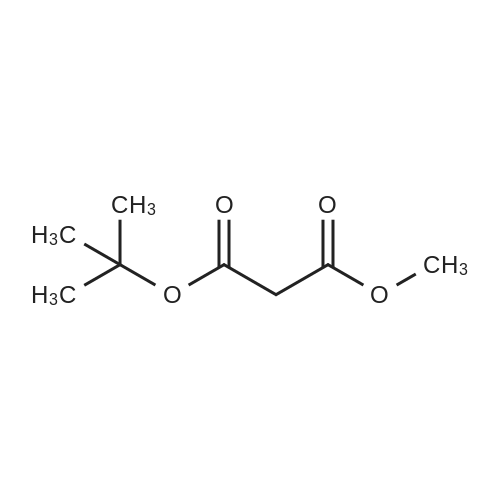 [ 42726-73-8 ]
[ 42726-73-8 ]
 [ 74-88-4 ]
[ 74-88-4 ]
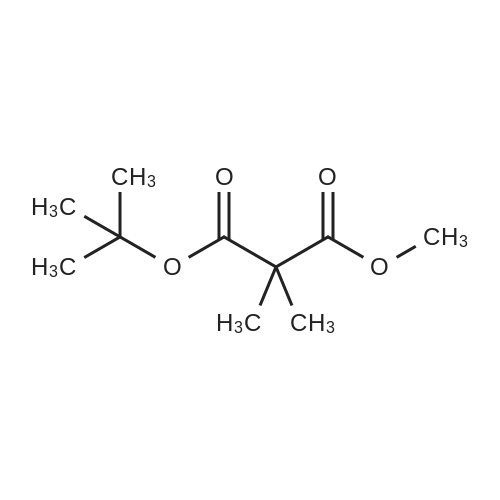 [ 85293-46-5 ]
[ 85293-46-5 ]
| Yield | Reaction Conditions | Operation in experiment |
|---|
| 94% | | To a suspension of 60 % wt. sodium hydride in mineral oil (1.56 g, 39.0 mmol) in tetrahydrofuran (40 mL) was added <strong>[42726-73-8]tert-butyl methyl malonate</strong> (3.50 g, 20.0 mmol) dropwise at 0 C. After stirring at this temperature for 30 minutes, iodomethane (5.54 g, 39.0 mmol) was added dropwise and it was continued to stir at room temperature foranother 5 hours. Then the mixture was quenched with water (30 mL) and extracted withethyl acetate (50 mL) twice. The combined organic layers were washed with brine (50mL), dried over Na2SO4() and filtered. The filtrate was concentrated under reducedpressure to give the title compound (3.80 g, 94 % yield) as brown oil. ?H NIVIR (300 1VIHz, CDC13) 3.72 (s, 3H), 1.44 (s, 9H), 1.40 (s, 6H). |
| 24.76 g | | Sodium hydride (15.6 g) was suspended in 300 mL of tetrahydrofuran under a nitrogen stream, and Compound1 (30 mL) was added dropwise over 30 minutes under ice cooling. After the mixture was stirred under ice cooling for 30minutes, methyl iodide (24.3 mL) was added dropwise, and the mixture was stirred at room temperature for 16 hours.To the reaction solution was added water, and the mixture was extracted with ethyl acetate. The extract was washedwith saturated brine, and dried over anhydrous magnesium sulfate. The solution was concentrated under reducedpressure to obtain Compound 2 (24.76 g). The obtained Compound 2 (24.76 g) was dissolved in tetrahydrofuran (320mL), 1M lithium hydroxide tri-tert-butoxyaluminum (300 mL) was added dropwise over 45 minutes under a nitrogenstream at room temperature, and the mixture was heated at reflux for 2 hours. To the reaction solution was addedsaturated brine, and the mixture was filtered through Celite and washed with ethyl acetate. The filtrate was concentratedunder reduced pressure, to the concentrated residue were added ethyl acetate and water, and the mixture was extractedwith ethyl acetate. The extract was washed with saturated brine, and dried over anhydrous magnesium sulfate. Afterthe solution was concentrated under reduced pressure, the obtained residue was distilled under reduced pressure toobtain Compound 3 (13.52 g).MS (m/z): 175 [M+H]+ |
| | The compound was synthesized in accordance with PCT International Application Publication No. WO 2007116922. Sodium hydride (60% in mineral oil; 2.0 g) was added to a cooled solution of <strong>[42726-73-8]tert-butyl methyl malonate</strong> (4 g) in THF (100 mL) at 0 C. under an atmosphere of Ar. The mixture was stirred at 0 C. for 10 min. MeI (3.2 mL) was added to the mixture and the stirring was continued for 3 h (by this time the mixture was at room temperature). Brine and EtOAc were added to the mixture, and the organic layer was separated, dried (Na2SO4), filtered and concentrated under vacuum to give the product (ca. 4.5 g), which was used directly in the next step. (1241) Solid lithium tri-tert-butoxy-aluminohydride (7.1 g, 28 mmol) was added portion-wise over 15 min to a solution of tert-butyl methyl 2,2-dimethyl-malonate (2.2 g) in THF (100 mL) under an atmosphere of Ar. The mixture was then heated to reflux and stirred overnight. After cooling to room temperature, a saturated solution of NH4Cl and EtOAc were added, and the aqueous and organic layers were separated. The organic layer was washed with H2O and brine, then dried (Na2SO4), filtered and concentrated under vacuum to provide a crude residue. The residue was purified by column chromatography on silica gel using EtOAc/hexanes (0:1 to 3:7) as eluent to give the product (14a) (900 mg) as an oil. 1H-NMR (300 MHz, CDCl3): delta 3.50 (d, J=5.1 Hz, 2H), 2.53 (t, J=6.5 Hz, 1H), 1.45 (s, 9H), 1.14 (s, 6H) |
| | The compound was synthesized in accordance with PCT International Application Publication No. WO 2007116922. 126 Sodium hydride (60percent in 127 mineral oil; 2.0 g) was added to a cooled solution of 128 <strong>[42726-73-8]tert-butyl methyl malonate</strong> (4 g) in 48 THF (100 mL) at 0° C. under an atmosphere of Ar. The mixture was stirred at 0° C. for 10 min. 129 MeI (3.2 mL) was added to the mixture and the stirring was continued for 3 h (by this time the mixture was at room temperature). Brine and 21 EtOAc were added to the mixture, and the organic layer was separated, dried (Na2SO4), filtered and concentrated under vacuum to give the product (ca. 4.5 g), which was used directly in the next step. |


 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping








