Alternatived Products of [ 5294-03-1 ] Product Details of [ 5294-03-1 ]
| CAS No. : | 5294-03-1 | MDL No. : | MFCD28452932 |
| Formula : |
C16H14 | Boiling Point : | - |
| Linear Structure Formula : | C2(C6H4CH3)2 | InChI Key : | SVIUEMOZHFTFEZ-UHFFFAOYSA-N |
| M.W : |
206.28
| Pubchem ID : | 10242091 |
| Synonyms : | |
Application In Synthesis of [ 5294-03-1 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 5294-03-1 ]
- 1
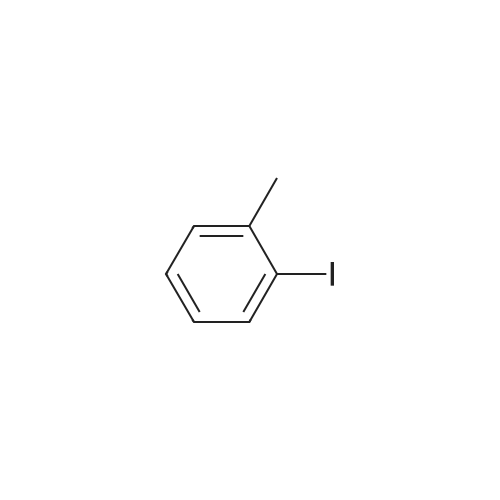 [ 615-37-2 ]
[ 615-37-2 ]
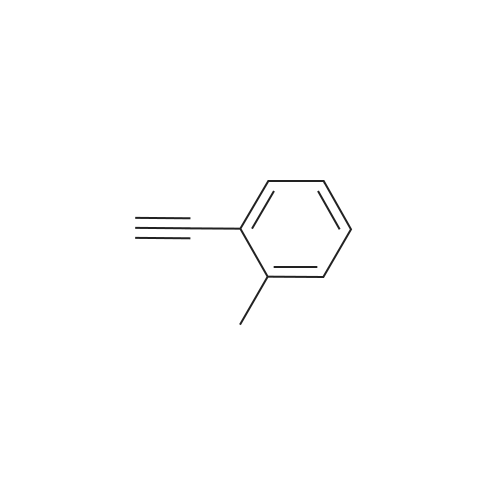 [ 766-47-2 ]
[ 766-47-2 ]
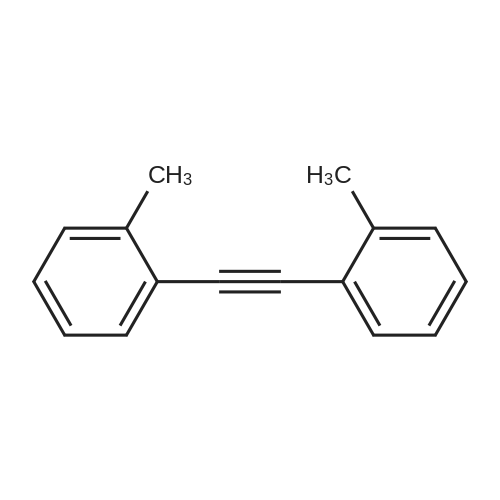 [ 5294-03-1 ]
[ 5294-03-1 ]
| Yield | Reaction Conditions | Operation in experiment |
|---|
| 75% | With C17H32Cl2N4OPd; potassium carbonate; In ethanol; at 80℃; for 1h; | General procedure: K2CO3 (2.5 × 10-4 mol, 2.5 equiv), aryl iodide (1.0 × 10-4 mol, 1.0 equiv), and alkyne (1.5 × 10-4 mol, 1.5 equiv) were mixed in a 10-mL vial, followed by addition of a solution of the selected catalyst (1 × 10-8 mol) in EtOH (1 mL). The vial was placed in a preheated oil bath at 80 C and stirred for 1 h. After cooling to 20-25 C, the reaction mixture was evaporated to dryness under a stream of dinitrogen followed by addition of 1.0 equiv of 1,2-dimethoxyethane as NMR internal standard, and extraction of the reaction mixture with three 0.20-mL portions of CDCl3. All fractions were joined and analyzed by 1H NMR spectroscopy. The product peak assignments were based on the authentic samples or on published dat, whereas quantifications were performed upon integration of the selected peak of the product relatively to the peak of the standard. |
| With C23H20Cl4N4OPd; potassium carbonate; In ethanol; at 80℃; for 4h; | General procedure: Selected base (1.5×10-4 mol, 1.5 equivs), aryl iodide (1.0×10-4 mol, 1.0 equiv) and terminal alkyne (1.0×10-4 mol, 1.0 equivs) were mixed in a 10-mL vial, followed by addition of a solution of the selected catalyst (1×10-8 mol) in EtOH (1 mL). The vial was placed in a pre heated oil bath at 80 C and stirred for 4 h. After cooling to ca. 25 C, the reaction mixture was evaporated to dryness under a stream of dinitrogen followed by addition of 1.0 equivof 1,2-dimethoxyethane (NMR internal standard), and extraction of the reaction mixture with three 0.20 mL portions of CDCl3. All fractions were joined and analyzed by 1H NMR spectroscopy. The product peak assignments were based on authentic samplesor on published data [56,62-68] (several sources were used for published compounds), while the structure of two new products,i.e. 3,4-dimethoxy-5-(phenylethynyl)benzaldehyde (derived from the coupling of 3-iodo-4,5-dimethoxybenzaldehyde with phenylacetylene) and 1-fluoro-2-[(2-methylphenyl)ethynyl]benzene (prepared from 2-fluorophenylacetylene and 2-iodotoluene were undoubtedly established using NMR spectroscopy, MS and elemental analyses (see Supplementary data). Quantifications were performed upon integration of the selected peak of the product in the 1H NMR relatively to the peak of the standard. |
Reference: [1]Chemistry - A European Journal,2021,vol. 27,p. 605 - 608 [2]Dalton Transactions,2014,vol. 43,p. 2098 - 2103 [3]Organic Letters,2009,vol. 11,p. 5594 - 5597 [4]Journal of Catalysis,2015,vol. 329,p. 449 - 456 [5]Organic Letters,2021,vol. 23,p. 4023 - 4028 [6]Dalton Transactions,2010,vol. 39,p. 9493 - 9504 [7]Journal of Organic Chemistry,2012,vol. 77,p. 5633 - 5645 [8]Angewandte Chemie - International Edition,2012,vol. 51,p. 9870 - 9872 [9]Chemical Communications,2014,vol. 50,p. 2488 - 2490 [10]Journal of Molecular Catalysis A: Chemical,2014,vol. 395,p. 162 - 171 [11]Organic Letters,2015,vol. 17,p. 536 - 539 [12]Angewandte Chemie - International Edition,2015,vol. 54,p. 10613 - 10617
Angew. Chem.,2015,vol. 127,p. 10759 - 10763,5 [13]Journal of Organic Chemistry,2016,vol. 81,p. 1733 - 1745 [14]Photochemical and Photobiological Sciences,2017,vol. 16,p. 1495 - 1501 [15]Organic and Biomolecular Chemistry,2019,vol. 17,p. 7679 - 7683 [16]Journal of the American Chemical Society,2019,vol. 141,p. 18970 - 18976 - 2
 [ 615-37-2 ]
[ 615-37-2 ]
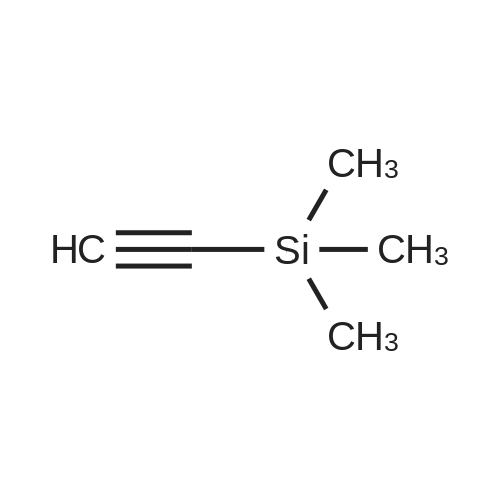 [ 1066-54-2 ]
[ 1066-54-2 ]
 [ 5294-03-1 ]
[ 5294-03-1 ]
| Yield | Reaction Conditions | Operation in experiment |
|---|
| 91% | | General procedure: 4-Iodoanisole (1mmol), TMSA (1.1mmol) and K2CO3 (2mmol) were added to a freshly prepared solution of PdNPs (5mL) in a 25mL round bottomed flask fitted with stopper. Then, the reaction mixture was stirred at 40C. The reaction progress was monitored by TLC, until complete consumption of aryl iodide. To the reaction mixture containing in situ formed 4-ethynylanisole the next batch of aryliodide (1mmol) was added and the reaction mixture was further allowed to stir until complete consumption of the arylacetylene. In this manner the targeted unsymmetrical diarylacetylene was formed. The detailed procedure is provided in the Supp. Info. Detailed procedure for synthesis of symmetrical diarylacetylenes is also mentioned in SI. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping








