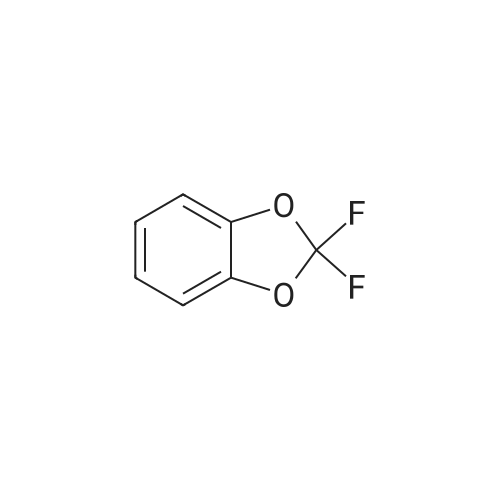
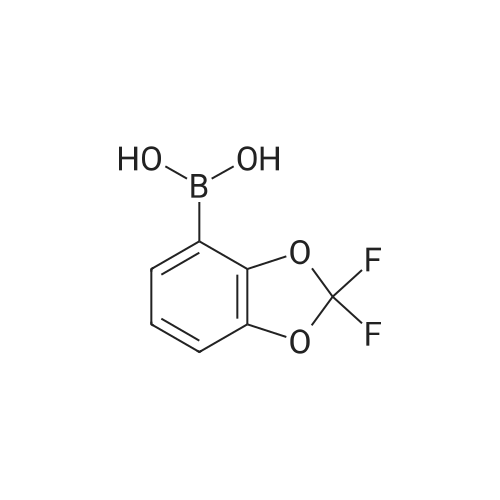
| | Intermediate 195(2,2-difluoro-l,3-benzodioxol-4-yl)boronic acid2,2-Difluoro-l,3-benzodioxole (960 mg, 6.1 mmol) was dissolved in THF (8 mL) and cyclohexane (4 mL) and the resulting solution cooled to -78 C. sec-BuLi 1.4M solution in cyclohexane (4.3 mL, 6.1 mmol) was added dropwise and the reaction mixture stirred for 1.5 hours at -78C. Trimethylborate (694 mg, 6.75 mmol) was added and the mixture was allowed to warm slowly to -30 C. The reaction mixture was quenched with a 2N solution of HCI and diluted with ethyl acetate. Two phases were separated and the organic layer was washed twice with brine, dried over Na2S04 and evaporated to dryness affording the title compound as yellow oil which was used in the next step without further purification. 1H-NMR (400 MHz, DMSO-d6 + D20): delta ppm 7.39 (1H, dd), 7.34 (1H, dd), 7.14 (t, 1H, 7=7.90 Hz). 19F-NMR (376 MHz, DMSO-d6 + D20): delta ppm -48.92. 13C-NMR (200 MHz, DMSO-d6 + D20): delta ppm 147.3, 142.8, 131.6 (t, 7=250.7 Hz), 130.1, 124.3, 112.0 |
| | 2,2-Difluoro-l,3-benzodioxole (960 mg, 6.1 mmol) was dissolved in THF (8 mL) and cyclohexane (4 mL) and the resulting solution cooled to -78 C. sec-BuLi 1.4M solution in cyclohexane (4.3 mL, 6.1 mmol) was added dropwise and the reaction mixture stirred for 1.5 hours at -78C. Trimethylborate (694 mg, 6.75 mmol) was added and the mixture was allowed to warm slowly to -30 C. The reaction mixture was quenched with a 2N solution of HCI and diluted with ethyl acetate. Two phases were separated and the organic layer was washed twice with brine, dried over Na2S04 and evaporated to dryness affording the title compound as yellow oil which was used in the next step without further purification. 1H-NM (400 M Hz, DMSO-d6 + D20): delta ppm 7.39 (1H, dd), 7.34 (1H, dd), 7.14 (t, 1H,7=7.90 Hz). 19F-NMR (376 MHz, DMSO-d6 + D20): delta ppm -48.92. 13C-NMR (200 M Hz, DMSO-d6 + D20): delta ppm 147.3, 142.8, 131.6 (t, 7=250.7 Hz), 130.1, 124.3, 112.0 |
| | 2,2-Difluoro-1,3-benzodioxole (960 mg, 6.1 mmol) was dissolved in THF (8 mL) and cyclohexane (4 mL) and the resulting solution cooled to -78 C. sec-BuLi 1.4M solution in cyclohexane (4.3 mL, 6.1 mmol) was added dropwise and the reaction mixture stirred for 1.5 hours at -78 C. Trimethylborate (694 mg, 6.75 mmol) was added and the mixture was allowed to warm slowly to -30 C. The reaction mixture was quenched with a 2N solution of HCl and diluted with ethyl acetate. Two phases were separated and the organic layer was washed twice with brine, dried over Na2SO4 and evaporated to dryness affording the title compound as yellow oil which was used in the next step without further purification. 1H-NMR (400 MHz, DMSO-d6+D2O): delta ppm 7.39 (1H, dd), 7.34 (1H, dd), 7.14 (t, 1H, J=7.90 Hz). 19F-NMR (376 MHz, DMSO-d6+D2O): delta ppm -48.92. 13C-NMR (200 MHz, DMSO-d6+D2O): delta ppm 147.3, 142.8, 131.6 (t, J=250.7 Hz), 130.1, 124.3, 112.0 |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping