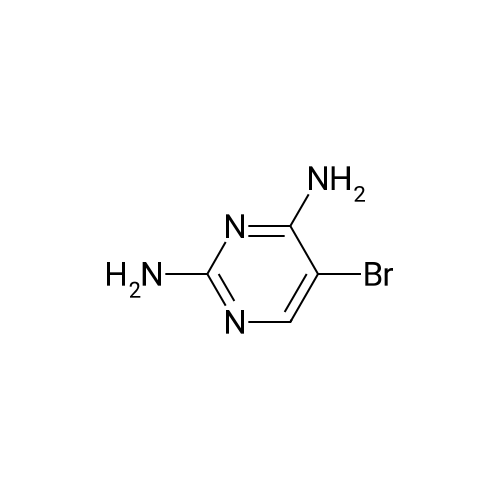
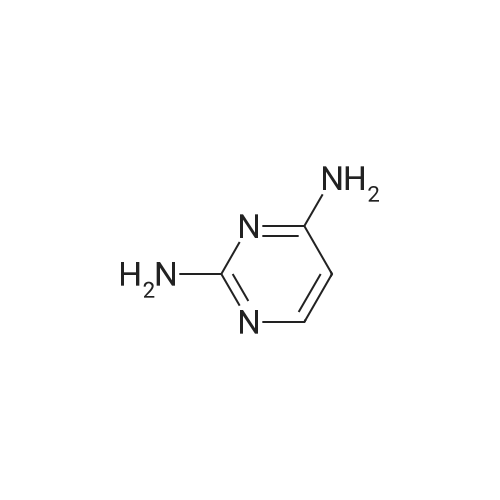
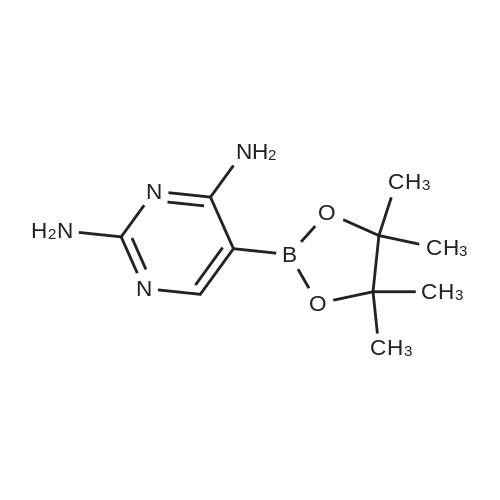
| 27% |
With potassium acetate;(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; In 1,4-dioxane; at 115℃; for 16h; |
Method 12; Synthesis of 5-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl)pyrimidine-2,4-diamine; [0252] To a dry 1 L flask was added 5-bromopyrimidine-2,4-diamine (30.0 g, 158.7 mmol), potassium acetate (45.8 g, 466.7 mmol), 4,4,5,5-tetramethyl-2-(4,4,5,5- tetramethyl-l,3,2-dioxaborolan-2-yl)-l,3,2-dioxaborolane (51.16g g, 202.2 mmol) and dioxane (50O mL). Argon was bubbled through the solution for 15 minutes, at which time l,l'-bis(diphenylphosphino)ferrocene palladium(II) chloride (2.53 g, 3.11 mmol) was added. The reaction was refluxed in a 115 0C oil bath for 16 hours under argon. After cooling to room temperature, the solid inorganic material was filtered, rinsed with EtOAc (1 L). The organic filtrate was concentrated in vacuo and to the resulting solid was added dichloromethane (1 L). After sonication the solid was filtered. The solid was the debrominated 2,4-diaminopyrimidine. The filtrate containing desired boronate ester was concentrated in vacuo. To this residue was added diethyl ether (10O mL). After <n="66"/>sonication, the solution was filtered, rinsed with additional diethyl ether (50 mL) and the solid obtained was dried under high vacuum to yield the desired 2,4-diaminopyrimidyl-5- boronate ester (10.13 g, 27%). By 1H NMR the material was a 4:1 mixture of 2,4- diaminopyrimidyl-5-boronate ester and 2,4-diaminopyrimidine byproduct. The material was used as is in subsequent Suzuki reactions LCMS (m/z): 155 (MH+ of boronic acid, deriving from in situ product hydrolysis on LC); 1H NMR (CDCI3+CD3OD): delta 8.16 (s, IH), 1.34 (s, 12H). |
|
With potassium acetate;dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; In 1,4-dioxane; at 115℃; for 16.25h;Heating / reflux; |
[0260] To a dry 1 L flask was added 5-bromopyrimidine-2,4-diamine (30.0 g,158.7 mmol), potassivim acetate (45.8 g, 466.7 rnmol), 4,4,5,5-tetramethyl-2-(4,4,5,5- tetramethyl-l,3,2-dioxaborolan-2-yl)-l,3,2-dioxaborolane (51.2 g, 202.2 mmol) and dioxane (50O mL). Argon was bubbled through the solution for 15 minutes, at which time l,r-bis(diphenylphosphino)ferrocene palladium(II) chloride (2.5 g, 3.11 mmol) was added. The reaction was refluxed in a 115 0C oil bath for 16 hours under argon. After cooling to room temperature, the solid inorganic material was filtered, rinsed with EtOAc (1 L). The organic filtrate was concentrated in vacuo and to the resulting solid was added dichloromethane (1 L). After sonication the solid was filtered. The solid was the debrominated 2,4-diaminopyrimidine. The filtrate containing desired boronate ester was concentrated in vacuo. To this residue was added diethyl ether (10O mL). After sonication, the solution was filtered, rinsed with additional diethyl ether (50 mL) and the solid obtained was dried under high vacuum to yield the desired 2,4-diaminopyrimidyl-5- boronate ester (10.13 g, 27%). By 1H NMR the material was a 4:1 mixture of 2,4- diaminopyrimidyl-5-boronate ester and 2,4-diaminopyrimidine byproduct. The material was used as is in subsequent Suzuki reactions. LCMS (m/z): 155 (MH+ of boronic acid, deriving from in situ product hydrolysis on LC). 1H lSIMR (CDCI3+CD3OD): delta 8.16 (s, IH), 1.34 (s, 12H). |
|
With dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; potassium acetate; In 1,4-dioxane; at 115℃; for 16h;Inert atmosphere; |
Synthesis of 5-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl)pyrimidine-2,4- diamine[00105] To a dry 1 L flask was added 5-bromopyrimidine-2,4-diamine (30.0 g,158.7 mmol), potassium acetate (45.8 g, 466.7 mmol), 4,4,5,5 -tetramethyl-2-(4,4,5, 5- tetramethyl-l ,3,2-dioxaborolan-2-yl)-l,3,2-dioxaborolane (51.2 g, 202.2 mmol) and dioxane (500 mL). Argon was bubbled through the solution for 15 minutes, at which time l,l'-bis(diphenylphosphino)ferrocene palladium(II) chloride (2.5 g, 3.11 mmol) was added. The reaction was refiuxed in a 115 C oil bath for 16 hours under argon. After cooling to room temperature, the solid inorganic material was filtered, rinsed with EtOAc (1 L). The organic filtrate was concentrated in vacuo and to the resulting solid was addeddichloromethane (1 L). After sonication the solid was filtered. The solid was the debrominated 2,4-diaminopyrimidine. The filtrate containing desired boronate ester was concentrated in vacuo. To this residue was added diethyl ether (100 mL). After sonication, the solution was filtered, rinsed with additional diethyl ether (50 mL) and the solid obtained was dried under high vacuum to yield the desired 2,4-diaminopyrimidyl-5 -boronate ester (10.13 g, 27%). By XH NMR the material was a 4: 1 mixture of 2,4-diaminopyrimidyl-5- boronate ester and 2,4-diaminopyrimidine byproduct. The material was used as is in subsequent Suzuki reactions. LCMS (m/z): 155 (MH+ of boronic acid, deriving from in situ product hydrolysis on LC). l NMR (CDC13+CD30D): delta 8.16 (s, 1H), 1.34 (s, 12H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping