| 97% |
With hydrogenchloride In methanol; diethyl ether |
12
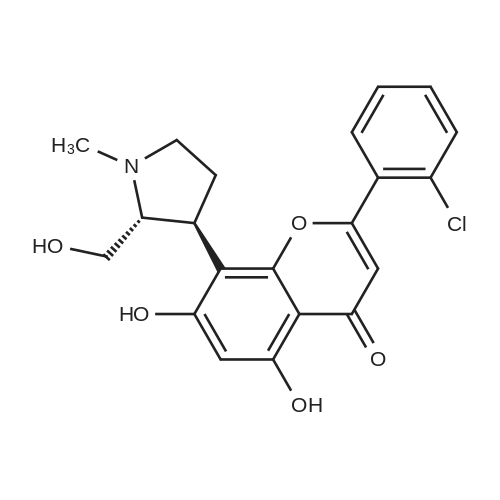
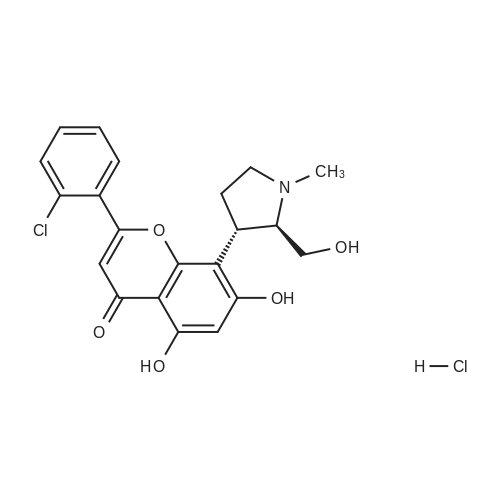
Example 12:(+)-£rans-2-(2-Chloro-phenyl)-5,7-dihydroxy-8-(2-hydroxymethyl-1 -methyl- pyrrolidin-3-yl)-chromen-4-one hydrochloride; (+)-^ans-2-(2-Chlorophenyl)-8-(2-hydroxymethyl-1 -methyl-pyrrolidin-3-yl)-5,7- dimethoxy-chromen-4-one (0.2 g, 0.48 mmol) was suspended in methanol (2 ml_) and ethereal HCI (5 ml_) was added. The suspension was stirred to get a clear solution. The solution was concentrated under reduced pressure to obtain the title compound. Yield: 0.21 g (97 %) [ ]D25 = +21.2° (c = 0.2, methanol)1H NMR (CD3OD, 300MHz): 7.80 (d, 1H), 7.60 (m, 3H), 6.53 (s, 1H), 6.37 (s, 1H), 4.23 (m, 1H), 3.89 (m, 2H), 3.63 (m, 1H), 3.59 (dd, 1H), 3.38 (m, 1H), 2.90 (s, 3H), 2.45 (m, 1H), 2.35 (m, 1H). MS (ES+): m/z 402 (M +1), free base. |
| 92% |
With hydrogenchloride In methanol; isopropyl alcohol at 25 - 50℃; |
11 Example 11 : (+)-frans-2-(2-Chlorophenyl)-5,7-dihydroxy-8-(2-hydroxymethyl-1 -methyl- pyrrolidin-3-yl)-chromen-4-one hydrochloride
77 g of the compound of example 10 was suspended in 462 mL of methanol at 25 - 30 °C into which 1 15.5 mL of isopropyl alcohol-HCI was added at 25 - 30 °C for 20 - 30 min. The reaction mixture was heated at 45 - 50 °C for 30 min. To this solution, 3.85 g of activated carbon was added and maintained at 45 - 50 °C for 15 - 30 min and filtered through 38.5 g celite bed. The bed was washed with 2 x 38.5 mL of hot methanol, the filtrate was collected and concentrated below 50 °C under vacuum till 2.5 volumes of reaction mixture was retained in the flask. The reaction mixture was further chased with 2 x 385 mL of diisopropyl ether by maintaining 2.5 volumes of reaction mixture retained in the flask. Finally 77 mL of methanol and 308 mL of diisopropyl ether were charged into the flask and stirred for 30 min at 45 - 50 °C. The reaction mixture was cooled to 25 - 30 °C over a period of 1 - 2 h and an additional volume of 77 mL methanol was added into the flask and stirred for 1 h at 25 - 30 °C. The reaction mixture was filtered and the bed was washed with a mixture of 77 mL of diisopropyl ether and 15.4 mL of methanol. The wet cake was stirred with 1 155 mL of n-heptane, the solvent was distilled out and the residue was cooled to 25-30 °C and filtered. The bed was washed with 77 mL of n-heptane and dried at 75 - 80 °C under vacuum (NLT 650 mm of Hg) for 48 - 72 h to afford the compound of formula (1 ). Yield: 78 g, 92 %; HPLC Purity: NLT 98 % a/a; IR (KBr): 3398, 3058, 1663, 1618, 1583, 1509, 855, 839, 772, 734, 657 cm"1 ; 1 H NMR(CDCI3, 300 MHz): δ 7.80(d, 1 H), 7.60(m, 3H), 6.53(s, 1 H), 6.37(s, 1 H), 4.23(m, 1 H), 3.89(m, 2H), 3.63(m, 1 H), 3.59(dd, 1 H), 3.38(m, 1 H), 2.90(s, 3H), 2.45(m, 1 H), 2.35(m, 1 H); Melting point (M.P): 188 - 192 °C; [a] D25 : +21 .3 0 (c = 0.2, methanol); MS(ES+): m/z 402 (M+1). |
| 64% |
With hydrogenchloride In methanol; isopropyl alcohol at 45 - 50℃; for 0.5h; |
10 Example 10: (+)-frans-2-(2-Chlorophenyl)-5,7-dihydroxy-8-(2-hydroxymethyl-1 -methyl- pyrrolidin-3-yl)-chromen-4-one hydrochloride
20 g of the compound of example 9 was suspended in a mixture of 120 mL of methanol and 30 mL of isopropyl alcohol-HCI and maintained at 45 - 50 °C for 30 min. To this solution, 1 g of activated carbon was added and maintained at 45 - 50 °C for 15 min and filtered through 10 g celite bed. The bed was washed with 20 mL of methanol, the filtrate was collected and concentrated below 50 °C under vacuum till 2.5 volumes of reaction mixture was retained in the flask. The reaction mixture was further chased with 2 x 100 mL of diisopropyl ether by maintaining 2.5 volumes of reaction mixture retained in the flask. Finally 20 mL of methanol and 80 mL of diisopropyl ether were charged into the flask and stirred for 30 min at 45 - 50 °C. The reaction mixture was cooled to 25 - 30 °C over a period of 1 - 2 h and an additional volume of 20 mL methanol was added into the flask and stirred for 1 h at 25 - 30 °C. The reaction mixture was filtered and the bed was washed with a mixture of 20 mL of diisopropyl ether and 4 mL of methanol. The wet cake was dried at 75-80 °C under vacuum for 72 h to yield the compound of formula (1) Yield: 14 g, 64%; HPLC Purity: 97% a/a; IR (KBr): 3398, 3058, 1663, 1618, 1583, 1509, 855, 839, 772, 734, 657 cm"1; 1H NMR(CDCI3, 300 MHz): δ 7.80(d, 1H), 7.60(m, 3H), 6.53(s, 1H), 6.37(s, 1H), 4.23(m, 1H), 3.89(m, 2H), 3.63(m, 1H), 3.59(dd, 1H), 3.38(m, 1H), 2.90(s, 3H), 2.45(m, 1H), 2.35(m, 1H); Melting point (M.P): 188-192 °C; [a] D25 : +21.30 (c = 0.2, methanol); MS(ES+): m/z 402 (M+1). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping