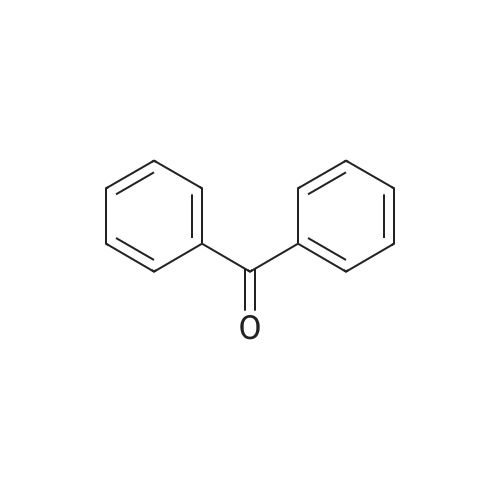
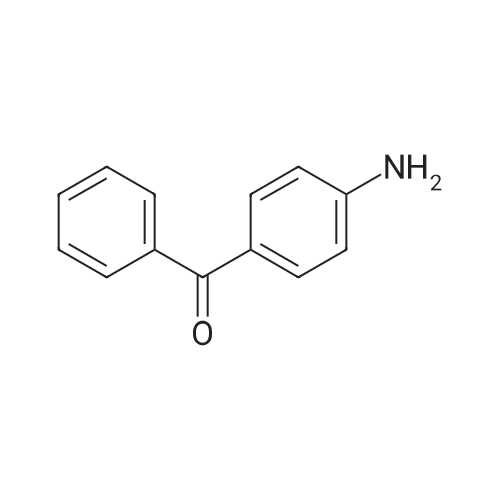
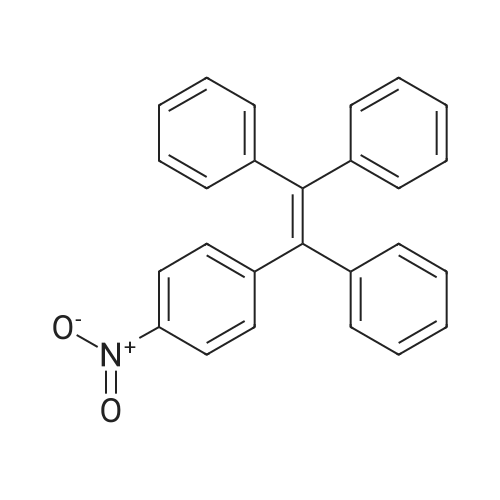
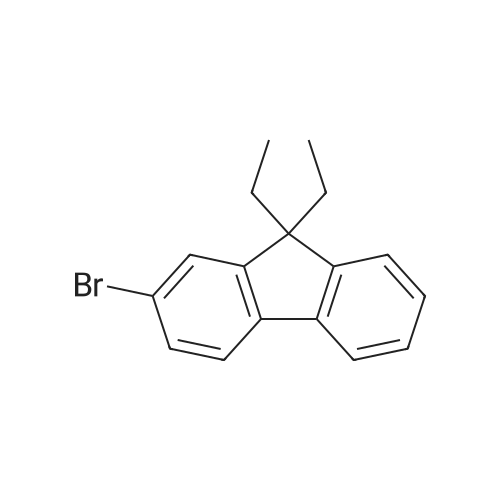
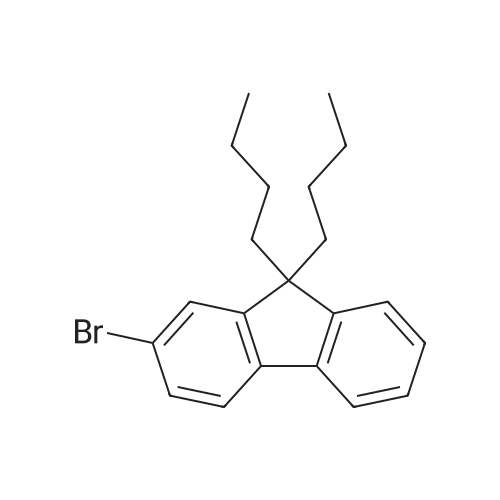
| 73.2% |
With tris-(dibenzylideneacetone)dipalladium(0); tri-tert-butyl phosphine; sodium t-butanolate In toluene for 48h; Inert atmosphere; Schlenk technique; Reflux; |
General procedure for the synthesis
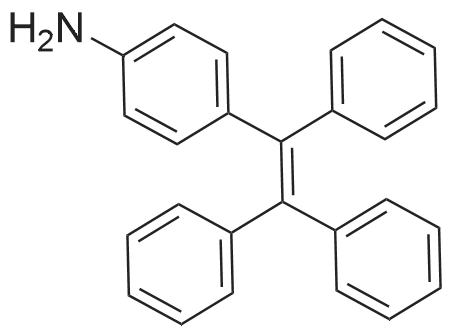
General procedure: Synthesis of compounds 1-3: A 50 mL two-necked round-bottom flask was evacuated under vacuum and then flushed with dry argon three times. Subsequently, compound I-1 (735.4 mg, 3.0 mmol) or I-2 (903.7 mg, 3.0 mmol) or I-3 (1072.0 mg, 3.0 mmol), Pd2(dba)3 (57.4 mg, 0.1 mmol), and t-BuONa (384.4 mg, 4.0 mmol) were added into the corresponding round-bottom flask. Next, toluene (30 ml), 4-(1,2,2-triphenylvinyl)aniline (347.5 mg, 1.0 mmol), and toluene solution of P(t-Bu)3 (2 ml) were added into the round-bottom flask. After that, the mixture was reuxed for 48 h and then cooled to room temperature. After completion of present reaction, the resulting mixture was extracted with dichloromethane and water, and then washed with brine three times. Organic layer was dried by anhydrous MgSO4, then the organic solvent was removed under vacuum, the residues were purified by column chromatography on silica gel with petroleum ether/dichloromethane (5:1) as eluent, affording the corresponding target product 1 (faint yellow solid), 2 (yellow solid) or 3 (light green solid) in a yield of 75.7%, 73.2% or 74.1%, respectively. 1: 1H NMR (500 MHz, CDCl3): δ (ppm) = 7.70 (d, J = 10 Hz, 2H), 7.64 (d, J = 10 Hz, 2H), 7.50 (d, J = 10 Hz, 2H), 7.35 (t, J = 7.5 Hz, 2H), 7.24 (s, 3H), 7.18-7.03 (m, 18H), 6.89-6.83 (m, 4H), 3.81 (s, 4H). 13C NMR (125 MHz, CDCl3): δ (ppm) = 146.8, 146.3, 144.5, 144.1, 143.8, 143.6, 143.0, 141.5, 140.7, 140.5, 137.7, 136.7, 132.2, 131.4, 131.4, 127.6, 127.6, 127.6, 126.8, 126.4, 126.3, 125.9, 124.9, 123.4, 122.5, 121.1, 120.3, 119.3, 36.9. EI-MS: m/z = 675.30[M]+. Anal. Calcd. For C52H37N: C, 92.41; H, 5.52, N, 2.07. Found: C, 92.50; H, 5.57; N, 2.01. 2: 1H NMR (500 MHz, CDCl3): δ (ppm) = 7.62 (d, J = 5 Hz, 2H), 7.56 (d, J = 10 Hz, 2H), 7.32-7.27 (m, 5H), 7.24-7.15 (m, 5H), 7.12-7.03 (m, 15H), 6.90-6.85 (m, 4H), 1.98-1.81 (m, 8H), 0.32 (t, J = 7.5 Hz, 12H). 13C NMR (125 MHz, CDCl3): δ (ppm) = 151.1, 149.7, 147.0, 146.3, 144.1, 143.8, 143.6, 141.3, 140.7, 140.4, 137.6, 136.4, 132.2, 131.4, 131.4, 127.6, 127.6, 126.8, 126.4, 126.4, 126.3, 126.2, 123.1, 122.7, 122.4, 120.1, 119.0, 118.9, 56.0, 32.7, 8.6. EI-MS: m/z = 787.40[M]+. Anal. Calcd. For C60H53N: C, 91.44; H, 6.78, N, 1.78. Found: C, 91.36; H, 6.85; N, 1.71. 3: 1H NMR (500 MHz, CDCl3): δ (ppm) = 7.61 (d, J = 5 Hz, 2H), 7.53 (d, J = 10 Hz, 2H), 7.30 (t, J = 5 Hz, 4H), 7.25 (s, 1H), 7.24-7.18 (m, 5H), 7.11-6.99 (m, 16H), 6.88 (s, 3H), 1.94-1.79 (m, 8H), 1.09-1.01 (m, 8H), 0.68-0.58 (m, 20H). 13C NMR (125 MHz, CDCl3): δ (ppm) = 151.9, 150.5, 146.8, 146.2, 144.1, 143.8, 143.6, 140.9, 140.8, 140.4, 137.7, 136.0, 132.1, 131.5, 131.4, 131.4, 127.6, 126.7, 126.5, 126.4, 126.3, 126.2, 123.0, 122.7, 122.4, 120.1, 119.0, 118.7, 54.9, 40.0, 26.0, 23.0, 13.9. EI-MS: m/z = 899.65[M]+. Anal. Calcd. For C68H69N: C, 90.72; H, 7.73, N, 1.56. Found: C, 90.79; H, 7.82; N, 1.51. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping