| 96.4% |
With water; sodium hydrogencarbonate; sodium hydroxide In tert-butyl methyl ether Reflux; Large scale; |
3b
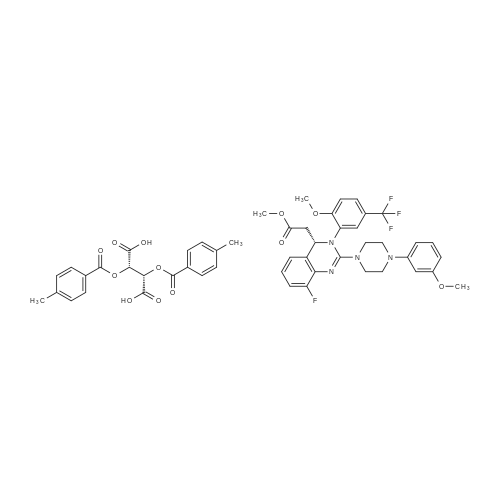
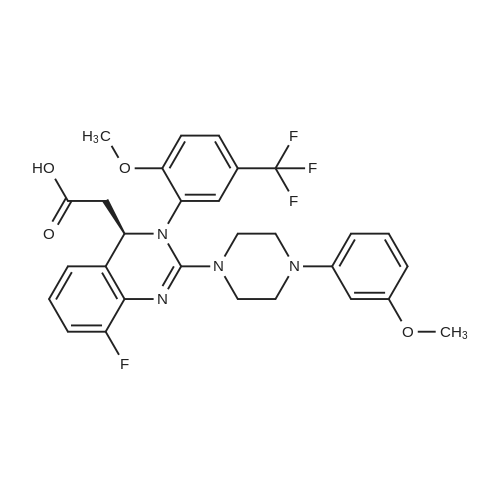
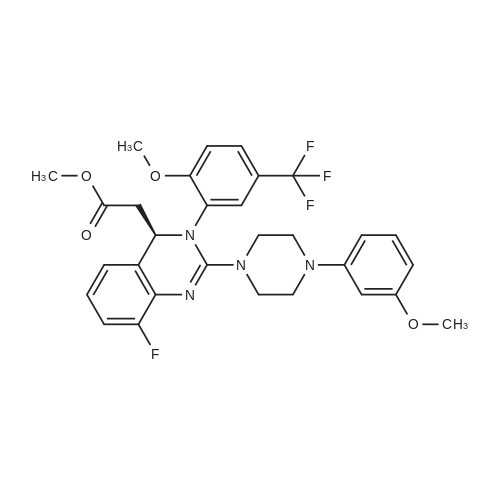
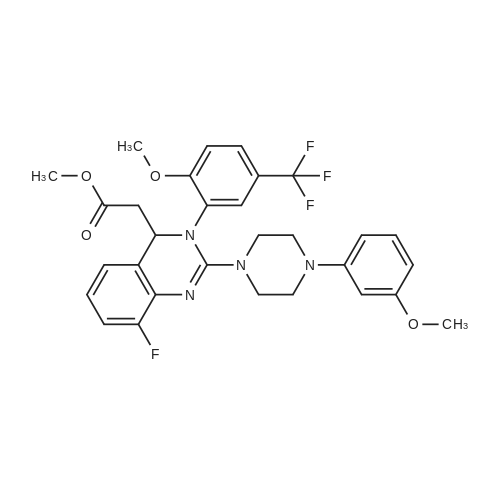
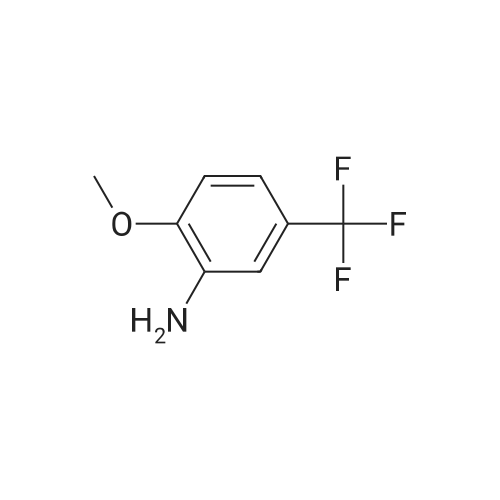
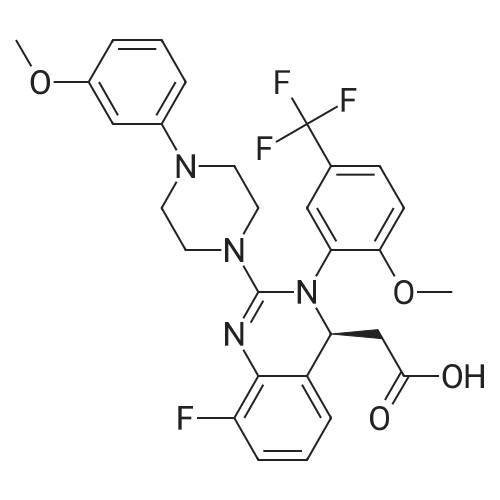
A mixture of (2S 3^-2,3-bis [(4-methylbenzoyl)oxy]succininc acid -{(45 8-fluoro-2-[4-(3- methoxyphenyl)piperazin- 1 -yl] -3 -[2-methoxy-5-(trifluoromethyl)phenyl] -3 ,4-dihydro- quinazolin-4-yl} acetic acid methyl ester (l :l-salt) (30,8 kg), sodium hydrogen carbonate (16.4 kg) and water (315 L) is stirred with MTBE (160 L). The phases obtained are separated and the organic phase is treated with 35 L of a 7% sodium hydrogen carbonate solution. The phases obtained are separated again and the organic phase is treated with 125 L of a 4% sodium hydroxide solution. The mixture is heated under reflux conditions. The solvent is distilled to run dry. The residual content of the reactor is stirred for further 5 h at 55 - 60°C. To the mixture MTBE (160 L) and water (65 L) is added under stirring at 22°C. The phases obtained are separated again and the organic phase is extracted with the aid of a 6% aqueous sodium chloride solution (30 L). The aqueous phases are reunited and stirred with water (25 L) and MTBE (160 L). The pH is adjusted to 6.5 with the aid of IN muriatic acid. The organic phase is separated, the solvent is gently distilled to run dry and the residue is dissolved in acetone (approximately 75 L). A change of the solvent is conducted towards acetone by means of 6 distillation steps of 130 L each. The product is subsequently precipitated by adding the residual solvent (approximately 60 L) under stirring conditions (61 rpm) in an excess of water (492 L) at room temperature. Followed by centrifugation, the isolated product is dried in a vacuum dryer equipped with a spiral crumbling roller at 40 to 80°C. By this procedure a yield of 16,5 kg of (S)-{8-fluoro-2-[4-(3-methoxyphenyl)piperazin- l-yl]-3-(2-memoxy-5-trifluormethylphenyl)-3,4-dihydroquina2olin-4-yl}acetic acid is obtained as amorphous compound corresponding to 96.4% in theory.1H NMR (300 MHz, d6-DMSO): δ = 7,53 (d,2J - 8,4, 1H), 7,41 (brs, 1H), 7,22 (d,2J = 8,5, 1H), 7,09-7,01 (m, 2H), 6,86 (m, 2H)56,45 (dd,2J = 8,2,3J = 1,8, 1H), 6,39-6,34 (m, 2H), 4,87 (t,2J= 7,3, 1H), 3,79 (brs, 3H), 3,68 (s, 3H), 3,50-3,38 (m, 4H), 2,96-2,75 (m, 5H), 2,45- 2,40 (m, 1H) ppm; MS (API-ES-neg.): m/z = 571 [(M-H), 100 %]; |
| 96.4% |
With water; sodium hydrogencarbonate; sodium hydroxide In tert-butyl methyl ether at 22℃; Reflux; Large scale; |
6A Example 6A (S)-{8-fluoro-2-[4-(3-methoxyphenyl)piperazine-1-yl]-3-(2-methoxy-5-trifluoromethylphenyl)-3,4-dihydroquinazoline-4-yl}acetic acid
A mixture of (2S,3S)-2,3-bis[(4-methylbenzoyl)oxy]succinic acid-{(4S)-8-fluoro-2-[4-(3-methoxyphenyl)piperazine-1-yl]-3-[2-methoxy-5-(trifluoromethyl)phenyl]-3,4-dihydroquinazoline-4-yl}acetic acid methyl ester (1:1 salt) (30.8 kg), sodium bicarbonate (16.4 kg), and water (315 l) is mixed with MTBE (160 l). The phases are separated and the organic phase is treated with 35 l of an approximately seven-percent aqueous solution of sodium bicarbonate. The phases are separated and the organic phase is added to 125 l of an approximately four-percent aqueous solution of sodium hydroxide. The reaction mixture is heated to reflux, the solution is evaporated to dryness, and the reactor contents are then agitated for an additional 5 h at 55-60° C. The reaction mixture is then added at approx. 22° C. to MTBE (160 l) and water (65 l) and agitated. The phases are separated and the organic phase is extracted with an approximately six-percent aqueous solution of sodium chloride (30 l). The combined aqueous phases are mixed with water (25 l) and MTBE (160 l) and the pH value is adjusted to approx. 6.5 with approx. 1 N of hydrochloric acid. The organic phase is separated, the solvent is evaporated to dryness, and the residue is dissolved in acetone (approx. 75 l). The solvent is changed to acetone (6 distillations with approx. 130 l each). The final product is then precipitated by adding water, isolated through centrifugation, and dried in a vacuum dryer. A total of 16.5 kg of (S)-{8-fluoro-2-[4-(3-methoxyphenyl)piperazine-1-yl]-3-(2-methoxy-5-trifluoromethylphenyl)-3,4-dihydroquinazoline-4-yl}acetic acid is thus obtained as an amorphous solid, corresponding to 96.4% of theory. 1H NMR (300 MHz, d6-DMSO): δ=7.53 (d, 2J=8.4, 1H), 7.41 (brs, 1H), 7.22 (d, 2J=8.5, 1H), 7.09-7.01 (m, 2H), 6.86 (m, 2H), 6.45 (dd, 2J=8.2, 3J=1.8, 1H), 6.39-6.34 (m, 2H), 4.87 (t, 2J=7.3, 1H), 3.79 (brs, 3H), 3.68 (s, 3H), 3.50-3.38 (m, 4H), 2.96-2.75 (m, 5H), 2.45-2.40 (m, 1H) ppm; MS (API-ES-neg.): m/z=571 [(M+H), 100%];HPLC (Method 1): RT=15.1 min; HPLC (Method 2): 99.8% e.e.; Pd (ICP): <1 ppm. |
| 96.4% |
With water; sodium hydrogencarbonate In tert-butyl methyl ether Large scale; |
6A (S)-{8-fluoro-2-[4-(3-methoxyphenyl)piperazine-1-yl]-3-(2-methoxy-5-trifluoromethylphenyl)-3,4-dihydroquinazoline-4-yl}acetic acid
A mixture of (2S,3S)-2,3-bis[(4-methylbenzoyl)oxy]succinic acid-{(4S)-8-fluoro-2-[4-(3-methoxyphenyl)piperazine-1-yl]-3-[2-methoxy-5-(trifluoromethyl)phenyl]-3,4-dihydroquinazoline-4-yl}acetic acid methyl ester (1:1 salt) (30.8 kg), sodium bicarbonate (16.4 kg), and water (315 l) is mixed with MTBE (160 l). The phases are separated and the organic phase is treated with 35 l of an approximately seven-percent aqueous solution of sodium bicarbonate. The phases are separated and the organic phase is added to 125 l of an approximately four-percent aqueous solution of sodium hydroxide. The reaction mixture is heated to reflux, the solution is evaporated to dryness, and the reactor contents are then agitated for an additional 5 h at 55-60° C. The reaction mixture is then added at approx. 22° C. to MTBE (160 l) and water (65 l) and agitated. The phases are separated and the organic phase is extracted with an approximately six-percent aqueous solution of sodium chloride (30 l). The combined aqueous phases are mixed with water (25 l) and MTBE (160 l) and the pH value is adjusted to approx. 6.5 with approx. 1 N of hydrochloric acid. The organic phase is separated out, the solvent is evaporated to dryness, and the residue is dissolved in acetone (approx. 75 l). The solvent is changed to acetone (6 distillations with approx. 130 l each). The final product is then precipitated by adding water, isolated through centrifugation, and dried in a vacuum dryer. A total of 16.5 kg of (S)-[8-fluoro-2-[4-(3-methoxyphenyl)piperazine-1-yl]-3-(2-methoxy-5-trifluoromethylphenyl)-3,4-dihydroquinazoline-4-yl]acetic acid is thus obtained as an amorphous solid, corresponding to 96.4% of theory. 1H NMR (300 MHz, d6-DMSO): δ=7.53 (d, 2J=8.4, 1H), 7.41 (brs, 1H), 7.22 (d, 2J=8.5, 1H), 7.09-7.01 (m, 2H), 6.86 (m, 2H), 6.45 (dd, 2J=8.2, 3J=1.8, 1H), 6.39-6.34 (m, 2H), 4.87 (t, 2J=7.3, 1H), 3.79 (brs, 3H), 3.68 (s, 3H), 3.50-3.38 (m, 4H), 2.96-2.75 (m, 5H), 2.45-2.40 (m, 1H) ppm; MS (API-ES-neg.): m/z=571 [(M+H), 100%]; |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping