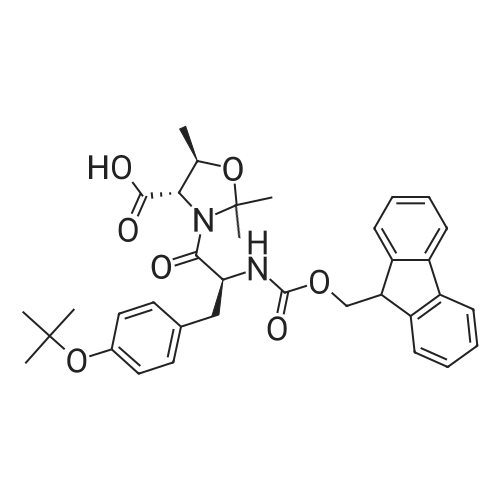
Alternatived Products of [ 911838-56-7 ]
Product Details of [ 911838-56-7 ]
CAS No. : 911838-56-7
MDL No. : MFCD11974987
Formula :
C33 H43 N3 O8
Boiling Point : -
Linear Structure Formula : -
InChI Key : JAHDJVRHKPYPNY-PHXCCWLDSA-N
M.W :
609.71
Pubchem ID : 75534997
Synonyms :
Chemical Name : (4S,5R)-3-((S)-2-((((9H-Fluoren-9-yl)methoxy)carbonyl)amino)-6-((tert-butoxycarbonyl)amino)hexanoyl)-2,2,5-trimethyloxazolidine-4-carboxylic acid
Calculated chemistry of [ 911838-56-7 ]
Physicochemical Properties
Num. heavy atoms : 44
Num. arom. heavy atoms : 12
Fraction Csp3 : 0.52
Num. rotatable bonds : 16
Num. H-bond acceptors : 8.0
Num. H-bond donors : 3.0
Molar Refractivity : 167.81
TPSA : 143.5 Ų
Pharmacokinetics
GI absorption : Low
BBB permeant : No
P-gp substrate : Yes
CYP1A2 inhibitor : No
CYP2C19 inhibitor : No
CYP2C9 inhibitor : Yes
CYP2D6 inhibitor : Yes
CYP3A4 inhibitor : Yes
Log Kp (skin permeation) : -6.61 cm/s
Lipophilicity
Log Po/w (iLOGP) : 3.87
Log Po/w (XLOGP3) : 4.8
Log Po/w (WLOGP) : 4.64
Log Po/w (MLOGP) : 2.17
Log Po/w (SILICOS-IT) : 3.9
Consensus Log Po/w : 3.88
Druglikeness
Lipinski : 2.0
Ghose : None
Veber : 2.0
Egan : 1.0
Muegge : 2.0
Bioavailability Score : 0.17
Water Solubility
Log S (ESOL) : -5.79
Solubility : 0.000989 mg/ml ; 0.00000162 mol/l
Class : Moderately soluble
Log S (Ali) : -7.55
Solubility : 0.0000174 mg/ml ; 0.0000000285 mol/l
Class : Poorly soluble
Log S (SILICOS-IT) : -7.32
Solubility : 0.0000289 mg/ml ; 0.0000000474 mol/l
Class : Poorly soluble
Medicinal Chemistry
PAINS : 0.0 alert
Brenk : 1.0 alert
Leadlikeness : 3.0
Synthetic accessibility : 5.68
Safety of [ 911838-56-7 ]
Application In Synthesis of [ 911838-56-7 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
Downstream synthetic route of [ 911838-56-7 ]
1
Fmoc-Gly-Wang resin
[ No CAS ]
[ 920519-31-9 ]
[ 68858-20-8 ]
[ 35661-60-0 ]
[ 957780-56-2 ]
[ 911838-56-7 ]
[ 71989-31-6 ]
[ 35661-40-6 ]
[ 71989-26-9 ]
[ 86060-81-3 ]
Nα-(9-fluorenylmethyloxycarbonyl)-Nγ-2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl-L-arginine
[ No CAS ]
Fmoc-L-thiazolidine-4-carboxylic acid
[ No CAS ]
Fmoc-Thz-AGY(tBu)C(Asm)-Tyr(tBu)-Thr(Ψ(Me,Me)pro)-R(Pbf)D(OtBu)LVY(tBu)K(Boc)D(OtBu)PA-R(Pbf)PK(Boc)IQ(Trt)-Lys(Boc)-Thr(Ψ(Me,Me)pro)-C(Asm)T(tBu)FK(Boc)E(OtBu)LVY(tBu)-Glu(OtBu)-Thr(Ψ(Me,Me)pro)-VR(Pbf)VPG-OH
[ No CAS ]
Yield Reaction Conditions Operation in experiment
520 mg
Stage #1: Fmoc-Gly-Wang resin With piperidine; 1,8-diazabicyclo[5.4.0]undec-7-ene In N,N-dimethyl-formamide Automated synthesizer; solid phase reaction;
Stage #2: Fmoc-Pro-OH Automated synthesizer; solid phase reaction;
Stage #3: (4S,5R)-3-(N-alpha-(9-Fluorenylmethyloxycarbonyl)-O-t-butyl-L-tyrosinyl)-2,2,5-trimethyloxazolidine-4-carboxylic acid; Fmoc-Val-OH; Fmoc-Leu-OH; Fmoc-Glu(OtBu)-Thr(Ψ(Me,Me)pro)-OH; Fmoc-Lys(Boc)-Thr(Ψ(Me,Me)-Pro)-OH; N-Fmoc L-Phe; Fmoc-Lys(tert-butoxycarbonyl); S-[(acetylamino)methyl]-N-[(9H-fluoren-9-ylmethoxy)carbonyl]-L-cysteine; Nα-(9-fluorenylmethyloxycarbonyl)-Nγ-2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl-L-arginine; Fmoc-L-thiazolidine-4-carboxylic acid Further stages;
2
[ 68858-20-8 ]
[ 35661-60-0 ]
[ 911838-56-7 ]
C16 H21 N2 O4 Pol
[ No CAS ]
C23 H27 NO5
[ No CAS ]
C23 H25 NO6
[ No CAS ]
C24 H27 NO6
[ No CAS ]
[ 71989-31-6 ]
[ 71989-38-3 ]
[ 71989-26-9 ]
[ 132388-59-1 ]
[ 132327-80-1 ]
Nle-Ile-Pro-Gly-Gly-Leu-Ser-Glu-Ala-Lys-Pro-Ala-Thr-Pro-Glu-Ile-Gln-Glu-Ile-Val-Asp-Lys-Val-Lys-Pro-Gln-Leu-Glu-Glu-Lys-Thr-Asn-Glu-Thr-Tyr-NHCH2 CCH
[ No CAS ]
Yield Reaction Conditions Operation in experiment
Stage #1: C16 H21 N2 O4 Pol; Fmoc-Tyr(tBu)-OH With N-ethyl-N,N-diisopropylamine; HATU In N,N-dimethyl-formamide for 2h; solid phase reaction;
Stage #2: With piperidine In N,N-dimethyl-formamide solid phase reaction;
Stage #3: Fmoc-Val-OH; Fmoc-Leu-OH; Fmoc-Lys(Boc)-Thr(Ψ(Me,Me)-Pro)-OH; C23 H27 NO5 ; C23 H25 NO6 ; C24 H27 NO6 ; Fmoc-Pro-OH; Fmoc-Lys(tert-butoxycarbonyl); L-Asn(Trt); Fmoc-L-Gln(Trt)-OH Further stages;
3
[ 68858-20-8 ]
[ 911838-56-7 ]
[ 35661-39-3 ]
C24 H27 NO6
[ No CAS ]
[ 1262308-49-5 ]
C25 H41 N2 O4 PolSi
[ No CAS ]
[ 71989-23-6 ]
[ 71989-38-3 ]
[ 71989-26-9 ]
[ 132388-59-1 ]
[ 132327-80-1 ]
Nα-(9-fluorenylmethyloxycarbonyl)-Nγ-2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl-L-arginine
[ No CAS ]
N3 Lys-Leu-Glu-Ala-Val-Gln-Tyr-Lys-Thr-Gln-Val-Val-Ala-Gly-Thr-Asn-Tyr-Tyr-Ile-Lys-Val-Arg-Ala-NHCH2 CCSi(iPr)3
[ No CAS ]
4
[ 1071446-05-3 ]
[ 955048-89-2 ]
[ 29022-11-5 ]
[ 68858-20-8 ]
[ 35661-60-0 ]
[ 911838-56-7 ]
[ 35661-39-3 ]
[ 71989-31-6 ]
[ 71989-14-5 ]
[ 71989-18-9 ]
[ 71989-23-6 ]
[ 71989-38-3 ]
[ 71989-26-9 ]
[ 51077-16-8 ]
[ 191348-16-0 ]
[ 132388-59-1 ]
[ 132327-80-1 ]
[ 7693-46-1 ]
[ 109425-51-6 ]
Nα-(9-fluorenylmethyloxycarbonyl)-Nγ-2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl-L-arginine
[ No CAS ]
1,3-thiazolidine-PVYLAAVLE-phosphotyrosine-LTAEILELAGNAARDNKKTRIIPRHLQL-1-formyl-2,3-dihydro-1H-1,3-benzodiazole-5-carboxamide
[ No CAS ]
Yield Reaction Conditions Operation in experiment
40%
Stage #1: 3-N-[(9H-fluoren-9-yl)methoxycarbonyl]-amino-4-aminobenzoic acid With benzotriazol-1-ol; O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; for 2h;
Stage #2: With piperidine In N,N-dimethyl-formamide at 20℃;
Stage #3: Fmoc-Leu-Thr(ΨMe,Me pro)-OH; N-(fluoren-9-ylmethoxycarbonyl)glycine; Fmoc-Val-OH; Fmoc-Leu-OH; Fmoc-Lys(Boc)-Thr(Ψ(Me,Me)-Pro)-OH; N-[(9H-fluoren-9-ylmethoxy)carbonyl]-L-alanine; Fmoc-Pro-OH; Fmoc-(tBu)Asp-OH; Fmoc-Glu(OtBu)-OH; Fmoc-Ile-OH; Fmoc-Tyr(tBu)-OH; Fmoc-Lys(tert-butoxycarbonyl); N-(tert-butoxycarbonyl)-(R)-thiazolidine-4-carboxylic acid; (S)-3-[4-(Benzyloxy-hydroxy-phosphoryloxy)-phenyl]-2-(9H-fluoren-9-ylmethoxycarbonylamino)-propionic acid; L-Asn(Trt); Fmoc-L-Gln(Trt)-OH; 4-Nitrophenyl chloroformate; Fmoc-His(Trt)-OH; Nα-(9-fluorenylmethyloxycarbonyl)-Nγ-2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl-L-arginine Further stages;
Reference:
[1]Jbara, Muhammad; Maity, Suman Kumar; Morgan, Michael; Wolberger, Cynthia; Brik, Ashraf
[Angewandte Chemie - International Edition, 2016, vol. 55, # 16, p. 4972 - 4976][Angew. Chem., 2016, vol. 128, # 16, p. 5056 - 5060,5]
5
N-tert-butyloxycarbonyl-S-trityl-L-cysteine
[ No CAS ]
[ 29022-11-5 ]
[ 68858-20-8 ]
[ 35661-60-0 ]
[ 911838-56-7 ]
[ 35661-39-3 ]
[ 71989-31-6 ]
[ 71989-33-8 ]
[ 71989-14-5 ]
[ 71989-18-9 ]
[ 71989-23-6 ]
[ 71989-26-9 ]
[ 71989-35-0 ]
[ 132388-59-1 ]
[ 132327-80-1 ]
Fmoc-Val-Thr(ox)-OH
[ No CAS ]
Nα-(9-fluorenylmethyloxycarbonyl)-Nγ-2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl-L-arginine
[ No CAS ]
CVRNDEELNKLLGRVTIAQGGVLPNIQSVLLPKKTESAKSAKSK-NH2
[ No CAS ]
Yield Reaction Conditions Operation in experiment
50%
Stage #1: Fmoc-Lys(tert-butoxycarbonyl) With 6-chloro-3-((dimethylamino)(dimethyliminio)methyl)-1H-benzo[d][1,2,3]triazol-3-ium-1-olatehexafluorophosphate(V); N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃;
Stage #2: With piperidine In N,N-dimethyl-formamide at 20℃;
Stage #3: N-tert-butyloxycarbonyl-S-trityl-L-cysteine; N-(fluoren-9-ylmethoxycarbonyl)glycine; Fmoc-Val-OH; Fmoc-Leu-OH; Fmoc-Lys(Boc)-Thr(Ψ(Me,Me)-Pro)-OH; N-[(9H-fluoren-9-ylmethoxy)carbonyl]-L-alanine; Fmoc-Pro-OH; Fmoc-Ser(tBu)-OH; Fmoc-(tBu)Asp-OH; Fmoc-Glu(OtBu)-OH; Fmoc-Ile-OH; Fmoc-Lys(tert-butoxycarbonyl); Fmoc-Thr(tBu)-OH; L-Asn(Trt); Fmoc-L-Gln(Trt)-OH; Fmoc-Val-Thr(ox)-OH; Nα-(9-fluorenylmethyloxycarbonyl)-Nγ-2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl-L-arginine Further stages;
Reference:
[1]Jbara, Muhammad; Maity, Suman Kumar; Morgan, Michael; Wolberger, Cynthia; Brik, Ashraf
[Angewandte Chemie - International Edition, 2016, vol. 55, # 16, p. 4972 - 4976][Angew. Chem., 2016, vol. 128, # 16, p. 5056 - 5060,5]
6
[ 29022-11-5 ]
[ 68858-20-8 ]
[ 35661-60-0 ]
[ 911838-56-7 ]
[ 35661-39-3 ]
Fmoc-N-Me-Cys(2-nitrobenzyl)-OH
[ No CAS ]
[ 71989-31-6 ]
[ 35661-40-6 ]
[ 71989-33-8 ]
[ 71989-18-9 ]
[ 71989-38-3 ]
[ 71989-26-9 ]
[ 71989-28-1 ]
[ 132388-59-1 ]
[ 132327-80-1 ]
[ 109425-51-6 ]
Nα-(9-fluorenylmethyloxycarbonyl)-Nγ-2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl-L-arginine
[ No CAS ]
MSGRGKQGGKTRAKAKTRSSRAGLQFPVGRVHRLLRKGNYAERVGAG-NH2
[ No CAS ]
Yield Reaction Conditions Operation in experiment
Stage #1: Fmoc-N-Me-Cys(2-nitrobenzyl)-OH With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate at 20℃; for 1h;
Stage #2: With piperidine In N,N-dimethyl-formamide at 20℃;
Stage #3: N-(fluoren-9-ylmethoxycarbonyl)glycine; Fmoc-Val-OH; Fmoc-Leu-OH; Fmoc-Lys(Boc)-Thr(Ψ(Me,Me)-Pro)-OH; N-[(9H-fluoren-9-ylmethoxy)carbonyl]-L-alanine; Fmoc-Pro-OH; N-Fmoc L-Phe; Fmoc-Ser(tBu)-OH; Fmoc-Glu(OtBu)-OH; Fmoc-Tyr(tBu)-OH; Fmoc-Lys(tert-butoxycarbonyl); N-[(9H-fluoren-9-ylmethoxy)carbonyl]-L-methionine; L-Asn(Trt); Fmoc-L-Gln(Trt)-OH; Fmoc-His(Trt)-OH; Nα-(9-fluorenylmethyloxycarbonyl)-Nγ-2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl-L-arginine
Reference:
[1]Jbara, Muhammad; Maity, Suman Kumar; Morgan, Michael; Wolberger, Cynthia; Brik, Ashraf
[Angewandte Chemie - International Edition, 2016, vol. 55, # 16, p. 4972 - 4976][Angew. Chem., 2016, vol. 128, # 16, p. 5056 - 5060,5]

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping