|
With tris-(dibenzylideneacetone)dipalladium(0); tri-tert-butyl phosphine; sodium tertiary butoxide In toluene at 100℃; |
Examples of Sub 2-1
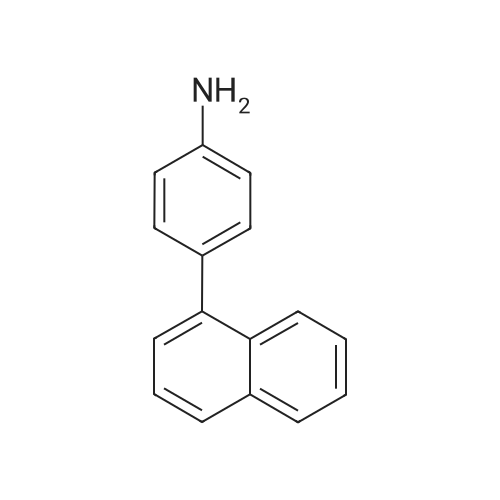
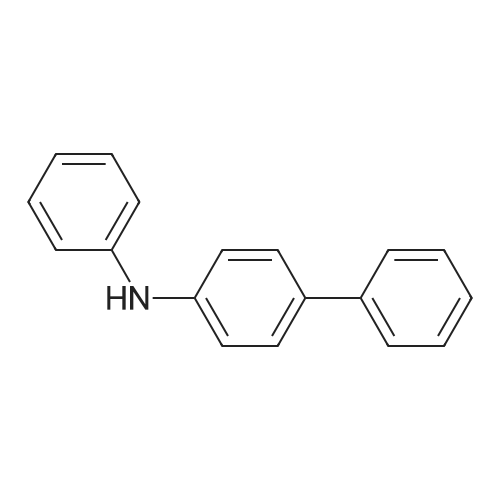
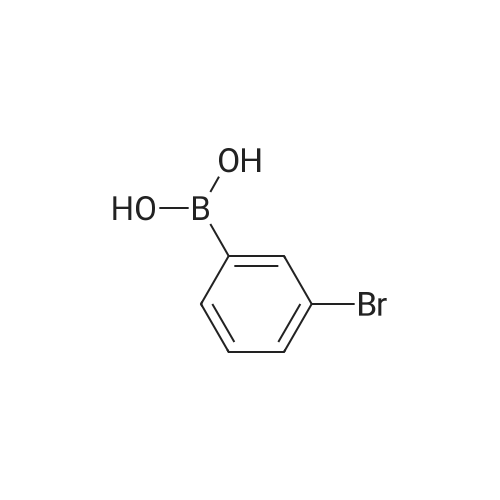
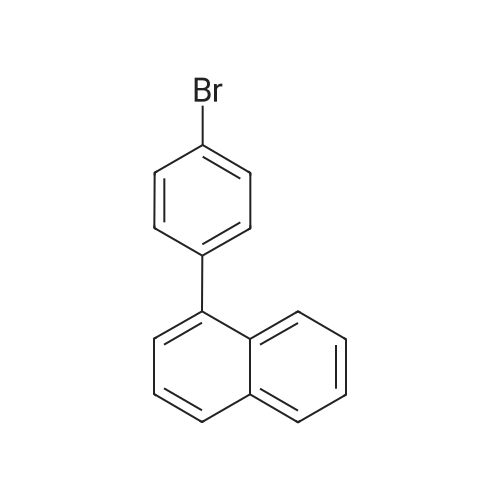
General procedure: Examples of Sub 2-1 (0055) (0056) After Aniline (15 g, 161.1 mmol), 1-bromonaphthalene (36.7 g, 177.2 mmol), Pd2(dba)3 (7.37 g, 8.05 mmol), P(t-Bu)3 (3.26 g, 16.1 mmol), NaOt-Bu (51.08 g, 531.5 mmol), and toluene (1690 mL) are added in a round bottom flask, stirring at 100° C. When the reaction is complete, the product was extracted with CH2Cl2 and water. The organic layer was dried over MgSO4 and concentrated, and then the product was separated by a silicagel column chromatography and recrystallized to obtain 25.4 g of product Sub 2-1 (yield: 72%). |
|
With tris-(dibenzylideneacetone)dipalladium(0); tri-tert-butyl phosphine; sodium tertiary butoxide In toluene at 100℃; |
1 Example of Sub 2-1
General procedure: A round bottom flask was charged with aniline (15 g, 161.1 mmol), 1-bromonaphthalene (36.7 g, 177.2 mmol), Pd2(dba)3 (7.37 g, 8.05 mmol), P(t-Bu)3 (3.26 g, 16.1 mmol), NaOt-Bu (51.08 g, 531.5 mmol), toluene (1690 mL) is added and the reaction proceeds at 100 ° C. After completion of the reaction, the reaction mixture was extracted with CH2Cl2 and water. The organic layer was dried over MgSO4 and concentrated. The resulting organic material was subjected to silicagel column and recrystallization to obtain 25.4 g of Sub 2-1. (Yield: 72%). |
|
With tris-(dibenzylideneacetone)dipalladium(0); tri-tert-butyl phosphine; sodium tertiary butoxide In toluene at 100℃; |
1 [Example of Sub 2-1]
General procedure: Aniline (15 g, 161.1 mmol), 1-bromonaphthalene (36.7 g, 177.2 mmol), Pd2 (dba) 3 (7.37g, 8.05 mmol), P (t-Bu) 3 (3.26 g, 16.1 mmol) in a round bottom flask ), NaOt-Bu (51.08 g, 531.5 mmol) and toluene (1690 mL) were added followed by reaction at 100 ° C. After the reaction was completed, the mixture was extracted with CH2Cl2 and water, the organic layer was dried over MgSO4 and concentrated, and the resulting organics were silicagel column and recrystallized to obtain 25.4 g of Sub 2-1. (Yield 72%) |
|
With tri-tert-butyl phosphine; Palladium(0) bis(dibenzylideneacetone); sodium tertiary butoxide In toluene at 100℃; |
1 [Example of Sub 2-1]
General procedure: Aniline (15 g, 161.1 mmol), 1-bromonaphthalene (36.7 g, 177.2 mmol), Pd2(dba)3(7.37 g, 8.05 mmol), P (t-Bu)3(3.26 g, 16.1) mmol), NaOt-Bu (51.08 g, 531.5 mmol) and toluene (1690 mL) were added followed by reaction at 100 ° C.After completion of the reaction, the mixturewasextracted withCH2Cl2and water, the organic layerwas dried overMgSO4and concentrated, and the resulting organics were purified by silicagel column and recrystallized to obtain 25.4 g of Sub 2-1.(Yield 72%) |
|
With tris-(dibenzylideneacetone)dipalladium(0); tri-tert-butyl phosphine; sodium tertiary butoxide In toluene at 100℃; |
1 [Example of Sub 2-1]
General procedure: Aniline (15 g, 161.1 mmol) in a round bottom flask,1-bromonaphthalene (36.7 g, 177.2 mmol),Pd 2 (dba) 3 (7.37 g, 8.05 mmol),P (t-Bu) 3 (3.26 g, 16.1 mmol),NaOt-Bu (51.08 g, 531.5 mmol),Add toluene (1690 mL) and proceed with the reaction at 100 ° C.After the reaction was completed, the mixture was extracted with CH2Cl2 and water, the organic layer was dried over MgSO4 and concentrated, and the resulting organics were purified by silicagel column and recrystallized to obtain 25.4 g of Sub 2-1. (Yield 72%) |
|
With tris-(dibenzylideneacetone)dipalladium(0); tri-tert-butyl phosphine; sodium tertiary butoxide In toluene at 100℃; |
1 [Example of Sub 2-1]
General procedure: To a round bottom flask was added Aniline (15 g, 161.1 mmol), 1-bromonaphthalene (36.7 g, 177.2 mmol), Pd2(dba)3 (7.37 g, 8.05 mmol), P(t-Bu)3 (3.26 g, 16.1 mmol), NaOt-Bu (51.08 g, 531.5 mmol) and toluene (1690 mL), and the reaction was performed at 100 ° C. After the reaction was completed, the mixture was extracted with CH2Cl2 and water, the organic layer was dried over MgSO4 and concentrated, and the resulting organic material was silicagel column and recrystallized to obtain 25.4 g of Sub 2-1. (Yield: 72%) |
|
With tris-(dibenzylideneacetone)dipalladium(0); 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl; sodium tertiary butoxide In toluene for 7h; Inert atmosphere; Reflux; |
11 Synthesis of intermediate C-1:
General procedure: Under the protection of nitrogen, toluene solvent (200mL), D-1 (3.72g, 40mmol), E-1 (8.28g, 40mmol), Pd2(dba)3(0.36g, 0.40mmol)were sequentially added to the reaction flask under the protection of nitrogen. , BINAP (0.74g, 1.20mmol) and sodium tert-butoxide (7.68g, 80mmol), stir to dissolve, and reflux for 7 hours under the protection of nitrogen. After the reaction is complete, cool the mixture to room temperature and filter through diatomaceous earth The filtrate was obtained, and the organic solvent was removed from the filtrate by distillation under reduced pressure. The obtained solid material was recrystallized with methanol to finally obtain Intermediate C-1 (7.19 g, yield 82%). |
|
With tris-(dibenzylideneacetone)dipalladium(0); tri-tert-butyl phosphine; sodium tertiary butoxide In toluene at 105℃; for 24h; Inert atmosphere; |
1 Example 1: Synthesis of intermediate B1:
General procedure: In a 250ml three-necked flask, under the protection of nitrogen, add 0.01mol of raw material 1-1, 0.012mol of raw material 2-1, and 150ml of toluene, stir and mix, and then add 5×10-5mol Pd2(dba)3, 5×10-5mol P( t-Bu)3, 0.03mol sodium tert-butoxide, heated to 105°C, refluxed for 24 hours, sampling point plate, the reaction is complete; naturally cooled to room temperature, filtered, the filtrate was rotary evaporated to no fraction, passed through a neutral silica gel column, Obtain the target product intermediate B1; HPLC purity 98.55%, yield 79.36%; |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping