| 80% |
With triethylamine In tetrahydrofuran at 60 - 65℃; for 20h; |
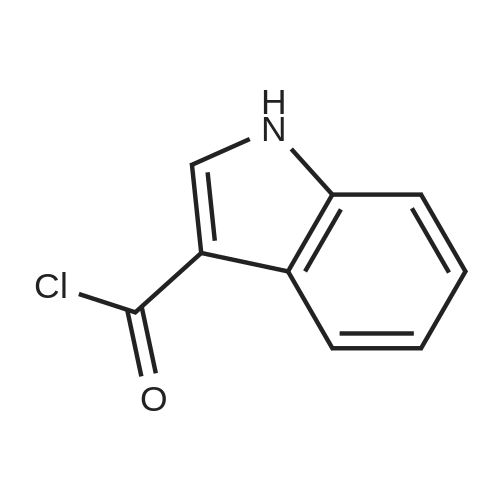
1.2 (2) Preparation of (IV) (Figure 5):
(III) (43.8 g, 0.31 mol), tetrahydrofuran (400 mL) and triethylamine (64.5 mL, 0.47 mol) were added to the reaction flask,The above-prepared solution of 3-indolyl chloride in tetrahydrofuran was added dropwise to the reaction flask,Bi completed,Rose to 60 ~ 65 ° C and stirred for 20h,TLC detection of the reaction process,After the reaction,The system was concentrated under reduced pressure to remove tetrahydrofuran,The residue was added with ethyl acetate (500mL) and water (250ml) were dissolved with stirring and transferred to a separatory funnel to separate,Points to the water layer,The organic layer was dried with anhydrous sodium sulfate,filter,The filtrate was concentrated to dryness under reduced pressure,Desopostan (IV) 70.6 g,Yield 80%; |
| 0.9 g |
Stage #1: 3-tropanol With n-butyllithium In tetrahydrofuran; hexane for 0.5h; Inert atmosphere;
Stage #2: Indole-3-carbonyl chloride In tetrahydrofuran; hexane for 16h; |
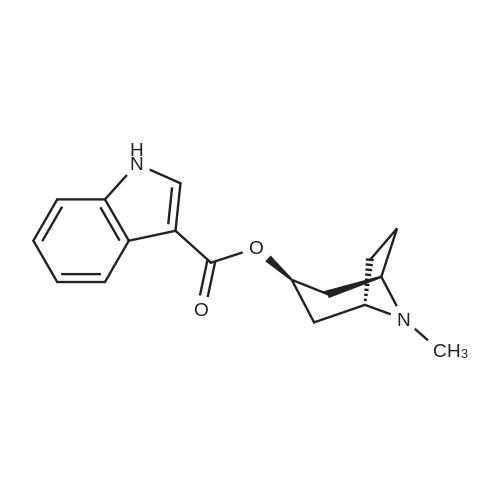
4 Synthesis of exo-8-Methyl-8-aza-bicyclo[3.2.1]octan-3-yl 1H-indole-3-carboxylate (Compound 4)
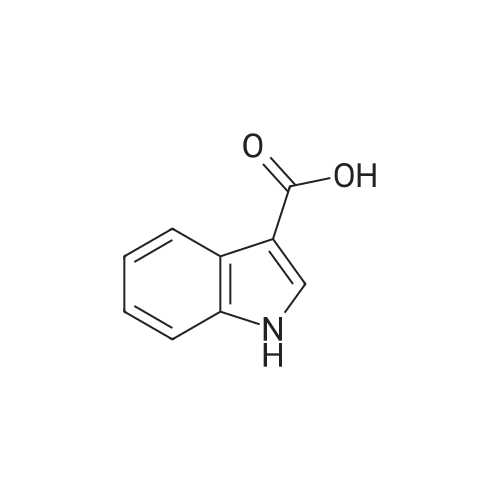
The synthesis of exo-8-methyl-8-aza-bicyclo[3.2.1]octan-3-yl 1H-indole-3-carboxylate (Compound 4) is illustrated in Synthesis Scheme 4 (). A solution of indole-3-carboxylic acid, 1 (1.5 g, 9.31 mmol) in dichloromethane (15 mL) at room temperature under argon atmosphere was treated with thionyl chloride (15 mL, 205.64 mmol) and stirred for 2 h under reflux. The solution was then concentrated to leave the acid chloride as a dark brown solid. The solid was then co-evaporated with dichloromethane (2*25 mL) and dried under vacuum to remove the volatile impurities. The dark brown solid was then dissolved in dry THF (15 mL). Meanwhile, on a separate flask, a solution of pseudotropine, 2 (1.45 g, 10.27 mmol) in dry THF (15 mL) at 5° C. under argon atmosphere was treated with n-butyl lithium (0.6 M in hexanes, 8.6 mL, 5.16 mmol) and stirred for 30 min. at the same temperature. A solution of the above acid chloride was added to the alkoxide solution at 5° C. dropwise. After completion of addition, the reaction mixture was allowed to warm to room temperature during which time a thick suspension was formed and stirred for another 16 h at room temperature. The reaction was monitored with TLC. After completion, the reaction mixture was evaporated under vacuum. The residue was dissolved in dichloromethane (100 mL), washed with water (3*50 mL), saturated brine (50 mL), dried over Na2SO4. The organic layer was filtered and evaporated under vacuum. The crude product was purified by flash column chromatography (neutral alumina) using a mixture of 4% MeOH in EtOAc as eluent to afford Cpd-4 (0.9 g, 34.0%) as a colorless solid. Rf: 0.2 (20% MeOH in CHCl3). 1H-NMR (CDCl3): δ 1.82-1.89 (m, 2H), 2.05-2.21 (m, 6H), 2.52 (s, 3H), 3.38 (bs, 2H), 5.44-5.51 (m, 1H), 7.26-7.31 (m, 2H), 7.43-7.47 (m, 1H), 8.05 (s, 1H), 8.21-8.27 (m, 1H), 11.45 (bs, 1H). LC-MS m/z: 285 [M+H]+. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping