Alternatived Products of [ 881-70-9 ]
Product Details of [ 881-70-9 ]
| CAS No. : | 881-70-9 |
MDL No. : | MFCD00026126 |
| Formula : |
C10H13NO3
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | - |
| M.W : |
195.22
|
Pubchem ID : | - |
| Synonyms : |
|
Safety of [ 881-70-9 ]
| Signal Word: | |
Class: | N/A |
| Precautionary Statements: | |
UN#: | N/A |
| Hazard Statements: | |
Packing Group: | N/A |
Application In Synthesis of [ 881-70-9 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 881-70-9 ]
- 1
-
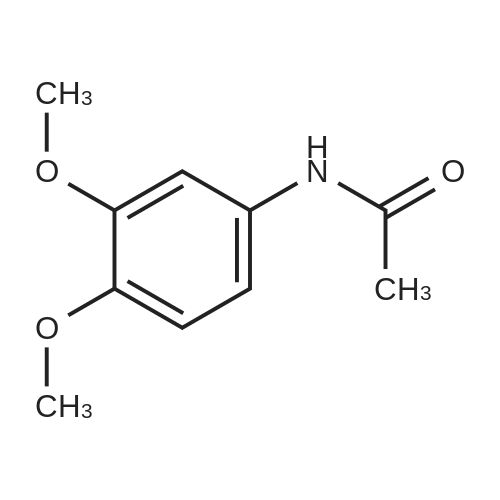 [ 881-70-9 ]
[ 881-70-9 ]

-
 [ 33513-42-7 ]
[ 33513-42-7 ]

-
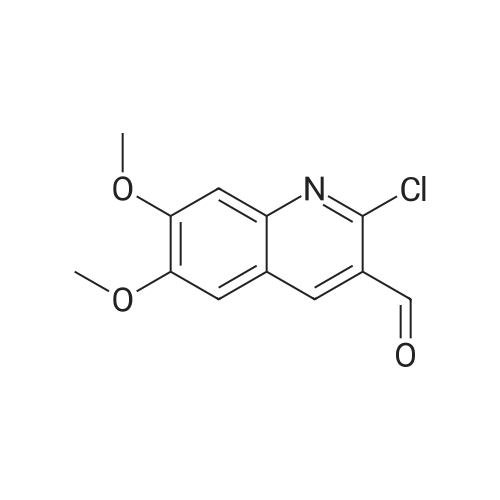 [ 68236-23-7 ]
[ 68236-23-7 ]
| Yield | Reaction Conditions | Operation in experiment |
| 72% |
With trichlorophosphate for 2h; Heating; |
|
| 56% |
With trichlorophosphate |
|
|
With trichlorophosphate In 1,2-dichloro-ethane r.t., 2 h, reflux, 3-4 h; |
|
|
With trichlorophosphate at 90℃; |
|
|
Stage #1: 3',4'-dimethoxyacetanilide; N,N-dimethyl-formamide With trichlorophosphate at 0 - 75℃; Inert atmosphere;
Stage #2: With water at 0 - 10℃; for 0.5h; Inert atmosphere; |
|
|
With trichlorophosphate at 75℃; for 3h; |
|
|
With trichlorophosphate for 16h; Reflux; |
|
|
With trichlorophosphate |
|
|
With trichlorophosphate |
|
|
With trichlorophosphate at 0 - 90℃; |
2.3. General procedure for preparation of 2-chloroquinoline-3-carbaldehydes (3a-3g)
General procedure: Dimethylformamide (3 mmol) was added to N-phenylacetamides(2a-2g, 1 mmol) in an ice bath, then phosphoryl chloride(15 mmol) was added dropwise to the mixture and was stirredat ice bath for 20 min, then the reaction proceeded at temperature80e90 C for 7e10 h. After completion of the reaction, ice is addedto the mixture and was stirred, then the formed precipitate wasfiltrated and recrystallized in ethanol [31].v |
|
With trichlorophosphate at 0 - 80℃; |
4.1.1. Synthesis of 2-oxoquinoline-3-carbaldehydes (1a-c) and 3-(hydroxymethyl)quinolinones (IVa-c) [34]
General procedure: These compounds were prepared by following the Meth-Cohnmethod. N,N-dimethylformamide (9.6 mL, 0.125 mol) was cooled to0 °C and phosphoryl chloride (32.2 mL, 0.35 mol) was added dropwisewith stirring. To this solution, the corresponding acetanilides IIa-c(0.05 mol) was added and the temperature of the reaction mixture wasraised to 80 °C during 8-19 h. The cooled reaction mixture was pouredinto ice-water (300 mL) and stirred for 10 min at 0-10 °C. The precipitateformed corresponding to 2-chloroquinoline-3-carbaldehydesIIIa-c was filtered off, washed with water (100 mL), dried, and recrystallizedfrom acetonitrile. A suspension of these aldehydes III(1 mmol) in 70% aqueous acetic acid (50 mL) was heated under refluxfor 24-72 h. Completion of the reaction was checked by TLC. Aftercooling the solid formed was filtered, washed well with water, driedand purified by recrystallization from DMF to obtain 1a-c. To a stirred solution of 2-oxo-1,2-dihydroquinoline-3-carbaldehydes 1a-c (1 mmol)in 15 mL methanol sodium borohydride (3 mmol) was slowly added atroom temperature. The progress reaction was monitored by TLC (20:1DCM:MeOH). After completion of the reaction (18 h), the solvent wasremoved under reduced pressure. To the residue formed, ice cold waterwas added and the obtained solid was filtered off and recrystallizedfrom MeOH to obtain 1d-f. To a stirred solution of 1d-f (1 mmol) inDCM (10 mL), a solution of SOCl2 (2 mL) in DCM (5 mL) was addeddropwise. After the addition was complete, four drops of DMF wasadded and the mixture was stirred for 2 h at reflux. The reaction progresswas monitored by the TLC (20:1 DCM:MeOH). After the solventwas removed under reduced pressure the compound 3-(chloromethyl)-quinolin-2(1H)-ones 1g-i were obtained as a syrup and further usedwithout purification. |

Reference:
[1]Meth-Cohn, Otto; Narine, Bramha; Tarnowski, Brian
[Journal of the Chemical Society. Perkin transactions I, 1981, p. 1520 - 1530]
[2]Metten, Bert; Smet, Mario; Boens, Noel; Dehaen, Wim
[Synthesis, 2005, # 11, p. 1838 - 1844]
[3]Gatti; Cavrini; Roveri; et al.
[European Journal of Medicinal Chemistry, 1984, vol. 19, # 5, p. 468 - 474]
[4]Location in patent: scheme or table
Herbert, Mark R.; Siegel, Dana L.; Staszewski, Lena; Cayanan, Charmagne; Banerjee, Urmi; Dhamija, Sangeeta; Anderson, Jennifer; Fan, Amy; Wang, Li; Rix, Peter; Shiau, Andrew K.; Rao, Tadimeti S.; Noble, Stewart A.; Heyman, Richard A.; Bischoff, Eric; Guha, Mausumee; Kabakibi, Ayman; Pinkerton, Anthony B.
[Bioorganic and Medicinal Chemistry Letters, 2010, vol. 20, # 19, p. 5718 - 5721]
[5]Location in patent: experimental part
Srihari, Ejjirothu; Kumar, Gangala Siva; Kumar, Chebolu Naga Sesha Sai Pavan; Seth, Ratnesh Kumar; Biswas, Sukla; Sridhar, Balasubramanian; Rao, Vaidya Jayathirtha
[Heterocyclic Communications, 2011, vol. 17, # 3-4, p. 111 - 119]
[6]Location in patent: scheme or table
Benameur, Ahmed; Boumoud, Taoues; Boumoud, Boudjemaa; Rhouati, Salah
[E-Journal of Chemistry, 2010, vol. 7, # 4, p. 1196 - 1199]
[7]Ramesh, Vadla; Ananda Rao, Boddu; Sharma, Pankaj; Swarna; Thummuri, Dinesh; Srinivas, Kolupula; Naidu; Jayathirtha Rao, Vaidya
[European Journal of Medicinal Chemistry, 2014, vol. 83, p. 569 - 580]
[8]Syniugin, Anatolii R.; Ostrynska, Olga V.; Chekanov, Maksym O.; Volynets, Galyna P.; Starosyla, Sergiy A.; Bdzhola, Volodymyr G.; Yarmoluk, Sergiy M.
[Journal of Enzyme Inhibition and Medicinal Chemistry, 2016, vol. 31, p. 160 - 169]
[9]Karthikeyan, Chandrabose; Amawi, Haneen; Viana, Arabela Guedes; Sanglard, Leticia; Hussein, Noor; Saddler, Maria; Ashby, Charles R.; Moorthy, N.S. Hari Narayana; Trivedi, Piyush; Tiwari, Amit K.
[Bioorganic and Medicinal Chemistry Letters, 2018, vol. 28, # 13, p. 2244 - 2249]
[10]Mirzaei, Salimeh; Hadizadeh, Farzin; Eisvand, Farhad; Mosaffa, Fatemeh; Ghodsi, Razieh
[Journal of Molecular Structure, 2020, vol. 1202]
[11]Abonia, Rodrigo; Andújar, Sebastián; Cobo, Justo; Enriz, Ricardo D.; Gutiérrez, Lucas; Insuasty, Daniel; Lima, Santiago; Marchal, Antonio; Nogueras, Manuel; Spiegel, Sarah; Vettorazzi, Marcela
[Bioorganic Chemistry, 2019]
- 2
-
 [ 75-36-5 ]
[ 75-36-5 ]

-
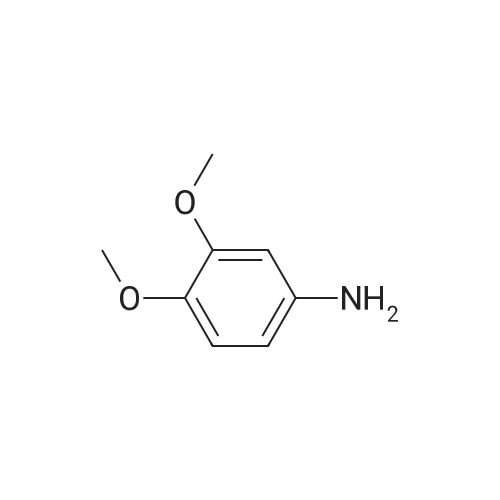 [ 6315-89-5 ]
[ 6315-89-5 ]

-
 [ 881-70-9 ]
[ 881-70-9 ]
| Yield | Reaction Conditions | Operation in experiment |
| 99% |
With triethylamine In dichloromethane at 0 - 20℃; |
Preparation of compound 1j
A solution of 3,4-dimethoxyaniline (248.7 mg, 1.62 mmol, 1.0 equiv) in DCM (5.0 mL) at 0 °Cwas added sequentially with Et3N (0.25 mL, 181.4 mg, 1.79 mmol, 1.1 equiv) and acetyl chloride(0.29 mL, 319 mg, 4.06 mmol, 2.5 equiv) which resulted in a dark solution. The reaction mixturewas then allowed to stir at room temperature. After 10 min, the reaction was complete as judgedby TLC. The reaction mixture was then added with water and the separated aqueous phase wasextracted with EtOAc (3x times). The combined organic phases were washed with sat. aq. NaCl,dried over anh. Na2SO4 and concentrated under reduced pressure to give 312.4 mg of analyticallypure N-(3,4-dimethoxyphenyl)acetamide (99%) as a purple solid. |
| 90% |
With pyridine for 0.916667h; Cooling with ice; |
|
| 57% |
With pyridine; dmap In dichloromethane at 0 - 20℃; Sealed tube; |
|
| 47% |
With triethylamine In dichloromethane at 20℃; |
|
| 39% |
With pyridine In dichloromethane at 0 - 20℃; Inert atmosphere; |
2. General procedure 1: preparation of 3,4-dimethoxyanilides 1
General procedure: To a solution of 3,4-dimethoxyaniline (0.77 g, 5.0 mmol) in CH2Cl2 (8.0 mL) was added pyridine (4.0 mL, 50 mmol) and benzoyl chloride (0.70 g, 5.0 mmol) in CH2Cl2 (2.0 mL) at 0 °C, and stirred at room temperature under argon. The reaction was monitored by TLC. The resulting mixture was quenched with 1M HCl, extracted with CH2Cl2 (3 x 20 mL). The combined organic layer was washed with brine, dried over MgSO4, and concentrated under vacuum. The crude compounds were purified with silica gel column chromatography (EtOAc : hexane = 1 : 2) and recrystallized from EtOAc/hexane to get the target anilide 1a (0.62 g, 48%). |
|
With triethylamine In dichloromethane for 1h; |
|
|
With triethylamine In chloroform at 0℃; for 1h; |
|
|
With triethylamine In chloroform at 0℃; for 1h; |
|
|
In dichloromethane at 20℃; for 1h; |
|
|
With triethylamine In dichloromethane at 0 - 20℃; for 10h; Inert atmosphere; |
|

Reference:
[1]Chaisan, Nattawadee; Kaewsri, Wilailak; Thongsornkleeb, Charnsak; Tummatorn, Jumreang; Ruchirawat, Somsak
[Tetrahedron Letters, 2018, p. 675 - 680]
[2]Iguchi, Daniela; Erra-Balsells, Rosa; Bonesi, Sergio M.
[Photochemical and Photobiological Sciences, 2016, vol. 15, # 1, p. 105 - 116]
[3]Bering, Luis; Vogt, Melina; Paulussen, Felix M.; Antonchick, Andrey P.
[Organic Letters, 2018, vol. 20, # 13, p. 4077 - 4080]
[4]Metten, Bert; Smet, Mario; Boens, Noel; Dehaen, Wim
[Synthesis, 2005, # 11, p. 1838 - 1844]
[5]Fujimoto, Shigenobu; Matsumoto, Kenji; Iwata, Takayuki; Shindo, Mitsuru
[Tetrahedron Letters, 2017, vol. 58, # 10, p. 973 - 976]
[6]Whiteley, Chris G.
[Bioorganic and Medicinal Chemistry, 2002, vol. 10, # 5, p. 1221 - 1227]
[7]Location in patent: scheme or table
Srihari, Ejjirothu; Kumar, Gangala Siva; Kumar, Chebolu Naga Sesha Sai Pavan; Seth, Ratnesh Kumar; Biswas, Sukla; Sridhar, Balasubramanian; Rao, Vaidya Jayathirtha
[Heterocyclic Communications, 2011, vol. 17, # 3-4, p. 111 - 119]
[8]Ramesh, Vadla; Ananda Rao, Boddu; Sharma, Pankaj; Swarna; Thummuri, Dinesh; Srinivas, Kolupula; Naidu; Jayathirtha Rao, Vaidya
[European Journal of Medicinal Chemistry, 2014, vol. 83, p. 569 - 580]
[9]Yan, Jun; Pang, Yanqing; Chen, Jie; Sheng, Jianfei; Hu, Jinhui; Huang, Ling; Li, Xingshu
[RSC Advances, 2015, vol. 5, # 119, p. 98527 - 98537]
[10]Shi, Yang; Liu, Jiahui; Yang, Yudong; You, Jingsong
[Chemical Communications, 2019, vol. 55, # 38, p. 5475 - 5478]
- 3
-
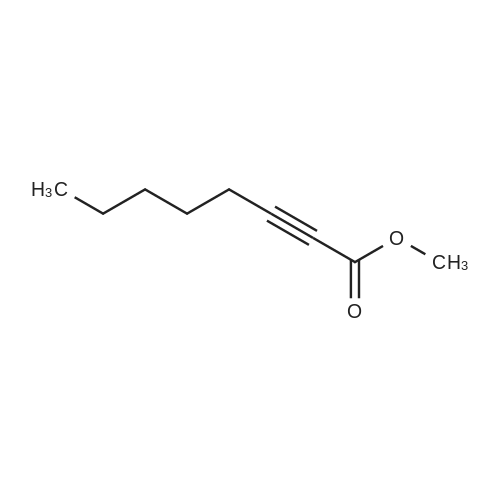 [ 111-12-6 ]
[ 111-12-6 ]

-
 [ 881-70-9 ]
[ 881-70-9 ]

-
 [ 1541180-81-7 ]
[ 1541180-81-7 ]
- 4
-
 [ 881-70-9 ]
[ 881-70-9 ]

-
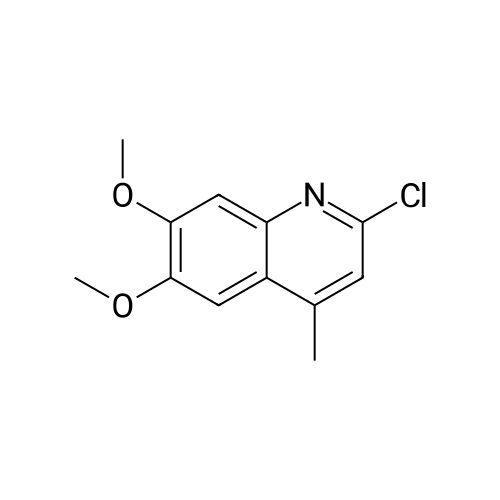 [ 697793-63-8 ]
[ 697793-63-8 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: [RhCl2(p-cymene)]2; silver hexafluoroantimonate; trimethylpyruvic acid / isopropyl alcohol / 24 h / 130 °C / Inert atmosphere; Glovebox
2: trichlorophosphate / acetonitrile / 4 h / 90 °C |
|
- 5
-
 [ 67-56-1 ]
[ 67-56-1 ]

-
 [ 62-44-2 ]
[ 62-44-2 ]

-
 [ 64-19-7 ]
[ 64-19-7 ]

-
 [ 881-70-9 ]
[ 881-70-9 ]

-
 [ 55274-58-3 ]
[ 55274-58-3 ]

-
 [ 103-90-2 ]
[ 103-90-2 ]

-
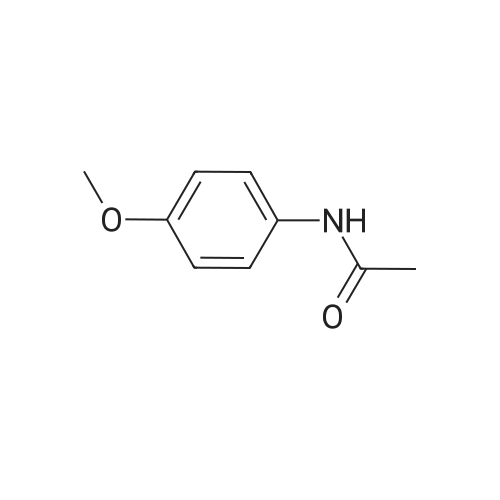 [ 51-66-1 ]
[ 51-66-1 ]
| Yield | Reaction Conditions | Operation in experiment |
| 33%Chromat.; 23%Chromat.; 11%Chromat.; 28%Chromat. |
With iron (III) meso-tetrakis (2,6-dichlorophenylporphyrin-beta-octabromo)chloride; 4-(trifluoromethyl)-1H-imidazole; 3-chloro-benzenecarboperoxoic acid; In acetonitrile; at 20℃; for 1.5h; |
General procedure: The conditions for a typical reaction were as follows:Fe(TDCPPBr8)Cl (2.7 mg, 1.67 × 10-3mmol) and imidazole(1.2 mg, 0.01674 mmol) were added to 500 L of 3:2 (v/v)acetonitrile/2-propanol in a 2-dram (7.39 mL) vial containinga screw cap. The vial was covered in aluminum foil, to protectfrom exposure to light, and the reaction was stirred at room temperature. After 30-min (to allow for axial ligation, if necessary),phenacetin (30 mg, 0.1674 mmol) was added to the reaction,followed by the slow addition of m-CPBA (77 wt%) (37.5 mg,0.1674 mmol) over a 1-h period. Following the addition of oxidant,the reaction was stirred for 24 h at room temperature, in theabsence of light. Typical catalyst/axial ligand/oxidant/substratemolar ratio was 1:5-10:100:100 (axial ligand: thiol or pKa? 9.0,5 mol%; N-heterocycle, 10 mol%) |
- 6
-
 [ 67-56-1 ]
[ 67-56-1 ]

-
 [ 62-44-2 ]
[ 62-44-2 ]

-
 [ 64-19-7 ]
[ 64-19-7 ]

-
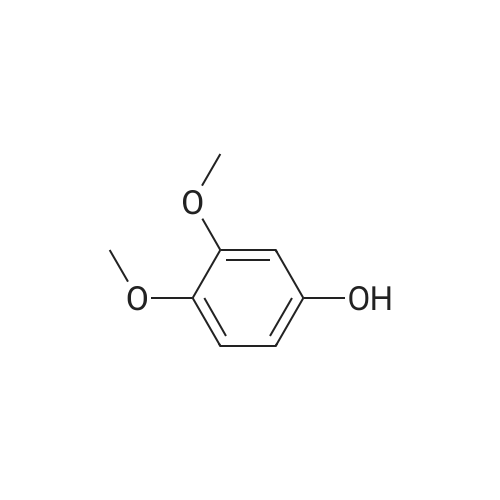 [ 2033-89-8 ]
[ 2033-89-8 ]

-
 [ 881-70-9 ]
[ 881-70-9 ]

-
 [ 55274-58-3 ]
[ 55274-58-3 ]

-
 [ 103-90-2 ]
[ 103-90-2 ]

-
 [ 51-66-1 ]
[ 51-66-1 ]
| Yield | Reaction Conditions | Operation in experiment |
| 7%Chromat.; 4%Chromat.; 51%Chromat.; 29%Chromat.; 9%Chromat. |
With iron (III) meso-tetrakis (2,6-dichlorophenylporphyrin-beta-octabromo)chloride; 3-chloro-benzenecarboperoxoic acid; In acetonitrile; at 20℃; for 1.5h; |
General procedure: The conditions for a typical reaction were as follows:Fe(TDCPPBr8)Cl (2.7 mg, 1.67 × 10-3mmol) and imidazole(1.2 mg, 0.01674 mmol) were added to 500 L of 3:2 (v/v)acetonitrile/2-propanol in a 2-dram (7.39 mL) vial containinga screw cap. The vial was covered in aluminum foil, to protectfrom exposure to light, and the reaction was stirred at room temperature. After 30-min (to allow for axial ligation, if necessary),phenacetin (30 mg, 0.1674 mmol) was added to the reaction,followed by the slow addition of m-CPBA (77 wt%) (37.5 mg,0.1674 mmol) over a 1-h period. Following the addition of oxidant,the reaction was stirred for 24 h at room temperature, in theabsence of light. Typical catalyst/axial ligand/oxidant/substratemolar ratio was 1:5-10:100:100 (axial ligand: thiol or pKa? 9.0,5 mol%; N-heterocycle, 10 mol%) |
- 7
-
 [ 67-56-1 ]
[ 67-56-1 ]

-
 [ 62-44-2 ]
[ 62-44-2 ]

-
 [ 64-19-7 ]
[ 64-19-7 ]

-
 [ 881-70-9 ]
[ 881-70-9 ]

-
 [ 55274-58-3 ]
[ 55274-58-3 ]

-
 [ 103-90-2 ]
[ 103-90-2 ]
| Yield | Reaction Conditions | Operation in experiment |
| 8%Chromat.; 33%Chromat.; 45%Chromat. |
With 2-methylimidazole; iron (III) meso-tetrakis (2,6-dichlorophenylporphyrin-beta-octabromo)chloride; 3-chloro-benzenecarboperoxoic acid; In acetonitrile; at 20℃; for 18h; |
General procedure: The conditions for a typical reaction were as follows:Fe(TDCPPBr8)Cl (2.7 mg, 1.67 × 10-3mmol) and imidazole(1.2 mg, 0.01674 mmol) were added to 500 L of 3:2 (v/v)acetonitrile/2-propanol in a 2-dram (7.39 mL) vial containinga screw cap. The vial was covered in aluminum foil, to protectfrom exposure to light, and the reaction was stirred at room temperature. After 30-min (to allow for axial ligation, if necessary),phenacetin (30 mg, 0.1674 mmol) was added to the reaction,followed by the slow addition of m-CPBA (77 wt%) (37.5 mg,0.1674 mmol) over a 1-h period. Following the addition of oxidant,the reaction was stirred for 24 h at room temperature, in theabsence of light. Typical catalyst/axial ligand/oxidant/substratemolar ratio was 1:5-10:100:100 (axial ligand: thiol or pKa? 9.0,5 mol%; N-heterocycle, 10 mol%) |
- 8
-
 [ 67-56-1 ]
[ 67-56-1 ]

-
 [ 62-44-2 ]
[ 62-44-2 ]

-
 [ 64-19-7 ]
[ 64-19-7 ]

-
 [ 2033-89-8 ]
[ 2033-89-8 ]

-
 [ 881-70-9 ]
[ 881-70-9 ]

-
 [ 55274-58-3 ]
[ 55274-58-3 ]

-
 [ 103-90-2 ]
[ 103-90-2 ]
| Yield | Reaction Conditions | Operation in experiment |
| 10%Chromat.; 7%Chromat.; 29%Chromat.; 36%Chromat. |
With iron (III) meso-tetrakis (2,6-dichlorophenylporphyrin-beta-octabromo)chloride; 2,4-Dimethylimidazole; 3-chloro-benzenecarboperoxoic acid; In acetonitrile; at 20℃; for 18h; |
General procedure: The conditions for a typical reaction were as follows:Fe(TDCPPBr8)Cl (2.7 mg, 1.67 × 10-3mmol) and imidazole(1.2 mg, 0.01674 mmol) were added to 500 L of 3:2 (v/v)acetonitrile/2-propanol in a 2-dram (7.39 mL) vial containinga screw cap. The vial was covered in aluminum foil, to protectfrom exposure to light, and the reaction was stirred at room temperature. After 30-min (to allow for axial ligation, if necessary),phenacetin (30 mg, 0.1674 mmol) was added to the reaction,followed by the slow addition of m-CPBA (77 wt%) (37.5 mg,0.1674 mmol) over a 1-h period. Following the addition of oxidant,the reaction was stirred for 24 h at room temperature, in theabsence of light. Typical catalyst/axial ligand/oxidant/substratemolar ratio was 1:5-10:100:100 (axial ligand: thiol or pKa? 9.0,5 mol%; N-heterocycle, 10 mol%) |
- 9
-
 [ 67-56-1 ]
[ 67-56-1 ]

-
 [ 62-44-2 ]
[ 62-44-2 ]

-
 [ 64-19-7 ]
[ 64-19-7 ]

-
 [ 881-70-9 ]
[ 881-70-9 ]

-
 [ 55274-58-3 ]
[ 55274-58-3 ]
| Yield | Reaction Conditions | Operation in experiment |
| 41%Chromat.; 44%Chromat. |
With 1H-imidazole; iron (III) meso-tetrakis (2,6-dichlorophenylporphyrin-beta-octabromo)chloride; 3-chloro-benzenecarboperoxoic acid; In acetonitrile; at 20℃; for 1.5h; |
General procedure: The conditions for a typical reaction were as follows:Fe(TDCPPBr8)Cl (2.7 mg, 1.67 × 10-3mmol) and imidazole(1.2 mg, 0.01674 mmol) were added to 500 L of 3:2 (v/v)acetonitrile/2-propanol in a 2-dram (7.39 mL) vial containinga screw cap. The vial was covered in aluminum foil, to protectfrom exposure to light, and the reaction was stirred at room temperature. After 30-min (to allow for axial ligation, if necessary),phenacetin (30 mg, 0.1674 mmol) was added to the reaction,followed by the slow addition of m-CPBA (77 wt%) (37.5 mg,0.1674 mmol) over a 1-h period. Following the addition of oxidant,the reaction was stirred for 24 h at room temperature, in theabsence of light. Typical catalyst/axial ligand/oxidant/substratemolar ratio was 1:5-10:100:100 (axial ligand: thiol or pKa? 9.0,5 mol%; N-heterocycle, 10 mol%) |
- 10
-
 [ 67-56-1 ]
[ 67-56-1 ]

-
 [ 62-44-2 ]
[ 62-44-2 ]

-
 [ 2033-89-8 ]
[ 2033-89-8 ]

-
 [ 881-70-9 ]
[ 881-70-9 ]

-
 [ 103-90-2 ]
[ 103-90-2 ]

-
 [ 51-66-1 ]
[ 51-66-1 ]
| Yield | Reaction Conditions | Operation in experiment |
| 4%Chromat.; 63%Chromat.; 20%Chromat.; 13%Chromat. |
With iron (III) meso-tetrakis (2,6-dichlorophenylporphyrin-beta-octabromo)chloride; thiophenol; 3-chloro-benzenecarboperoxoic acid; In acetonitrile; at 20℃; for 1.5h; |
General procedure: The conditions for a typical reaction were as follows:Fe(TDCPPBr8)Cl (2.7 mg, 1.67 × 10-3mmol) and imidazole(1.2 mg, 0.01674 mmol) were added to 500 L of 3:2 (v/v)acetonitrile/2-propanol in a 2-dram (7.39 mL) vial containinga screw cap. The vial was covered in aluminum foil, to protectfrom exposure to light, and the reaction was stirred at room temperature. After 30-min (to allow for axial ligation, if necessary),phenacetin (30 mg, 0.1674 mmol) was added to the reaction,followed by the slow addition of m-CPBA (77 wt%) (37.5 mg,0.1674 mmol) over a 1-h period. Following the addition of oxidant,the reaction was stirred for 24 h at room temperature, in theabsence of light. Typical catalyst/axial ligand/oxidant/substratemolar ratio was 1:5-10:100:100 (axial ligand: thiol or pKa? 9.0,5 mol%; N-heterocycle, 10 mol%) |
- 11
-
 [ 67-56-1 ]
[ 67-56-1 ]

-
 [ 62-44-2 ]
[ 62-44-2 ]

-
 [ 2033-89-8 ]
[ 2033-89-8 ]

-
 [ 881-70-9 ]
[ 881-70-9 ]

-
 [ 103-90-2 ]
[ 103-90-2 ]

-
 [ 106-51-4 ]
[ 106-51-4 ]
| Yield | Reaction Conditions | Operation in experiment |
| 15%Chromat.; 34%Chromat.; 39%Chromat.; 7%Chromat. |
With iron (III) meso-tetrakis (2,6-dichlorophenylporphyrin-beta-octabromo)chloride; phenylmethanethiol; 3-chloro-benzenecarboperoxoic acid; In acetonitrile; at 20℃; for 1.5h; |
General procedure: The conditions for a typical reaction were as follows:Fe(TDCPPBr8)Cl (2.7 mg, 1.67 × 10-3mmol) and imidazole(1.2 mg, 0.01674 mmol) were added to 500 L of 3:2 (v/v)acetonitrile/2-propanol in a 2-dram (7.39 mL) vial containinga screw cap. The vial was covered in aluminum foil, to protectfrom exposure to light, and the reaction was stirred at room temperature. After 30-min (to allow for axial ligation, if necessary),phenacetin (30 mg, 0.1674 mmol) was added to the reaction,followed by the slow addition of m-CPBA (77 wt%) (37.5 mg,0.1674 mmol) over a 1-h period. Following the addition of oxidant,the reaction was stirred for 24 h at room temperature, in theabsence of light. Typical catalyst/axial ligand/oxidant/substratemolar ratio was 1:5-10:100:100 (axial ligand: thiol or pKa? 9.0,5 mol%; N-heterocycle, 10 mol%) |
- 12
-
 [ 67-56-1 ]
[ 67-56-1 ]

-
 [ 62-44-2 ]
[ 62-44-2 ]

-
 [ 2033-89-8 ]
[ 2033-89-8 ]

-
 [ 881-70-9 ]
[ 881-70-9 ]

-
 [ 103-90-2 ]
[ 103-90-2 ]
| Yield | Reaction Conditions | Operation in experiment |
| 16%Chromat.; 15%Chromat.; 66%Chromat. |
With iron (III) meso-tetrakis (2,6-dichlorophenylporphyrin-beta-octabromo)chloride; thiophenol; 3-chloro-benzenecarboperoxoic acid; In acetonitrile; at 20℃; for 18h; |
General procedure: The conditions for a typical reaction were as follows:Fe(TDCPPBr8)Cl (2.7 mg, 1.67 × 10-3mmol) and imidazole(1.2 mg, 0.01674 mmol) were added to 500 L of 3:2 (v/v)acetonitrile/2-propanol in a 2-dram (7.39 mL) vial containinga screw cap. The vial was covered in aluminum foil, to protectfrom exposure to light, and the reaction was stirred at room temperature. After 30-min (to allow for axial ligation, if necessary),phenacetin (30 mg, 0.1674 mmol) was added to the reaction,followed by the slow addition of m-CPBA (77 wt%) (37.5 mg,0.1674 mmol) over a 1-h period. Following the addition of oxidant,the reaction was stirred for 24 h at room temperature, in theabsence of light. Typical catalyst/axial ligand/oxidant/substratemolar ratio was 1:5-10:100:100 (axial ligand: thiol or pKa? 9.0,5 mol%; N-heterocycle, 10 mol%) |
- 13
-
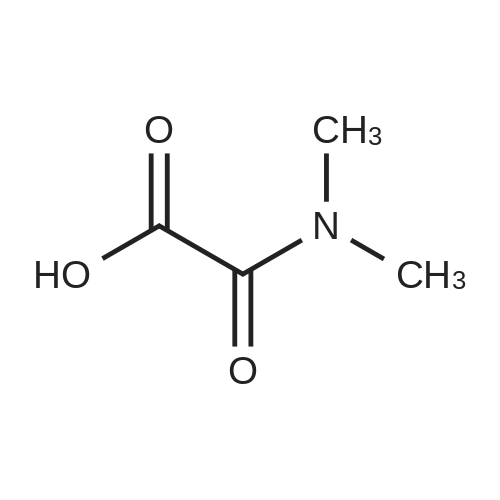 [ 32833-96-8 ]
[ 32833-96-8 ]

-
 [ 881-70-9 ]
[ 881-70-9 ]

-
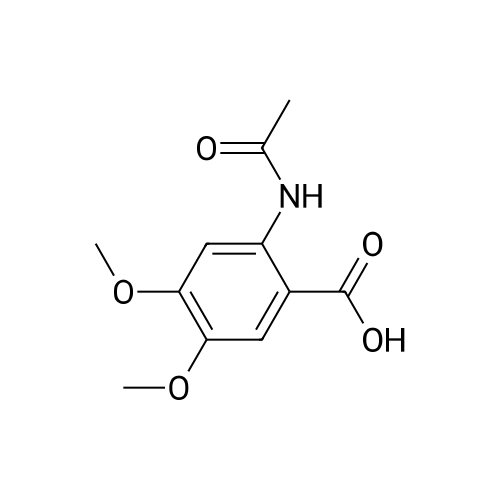 [ 145352-75-6 ]
[ 145352-75-6 ]
- 14
-
 [ 881-70-9 ]
[ 881-70-9 ]

-
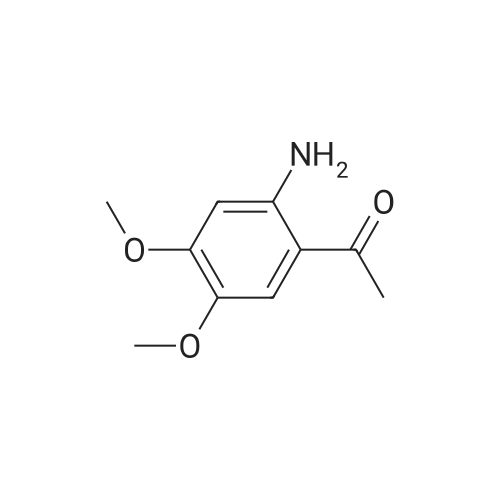 [ 4101-30-8 ]
[ 4101-30-8 ]

-
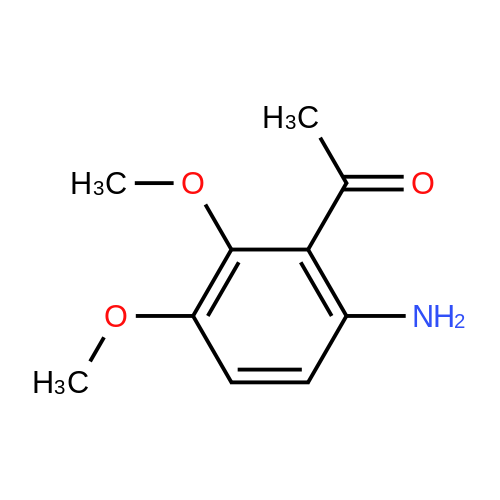 [ 98300-41-5 ]
[ 98300-41-5 ]
| Yield | Reaction Conditions | Operation in experiment |
| 1: 73%
2: 17% |
With brij 35 In water at 25℃; Irradiation; Micellar solution; Inert atmosphere; regioselective reaction; |
|
- 15
-
 [ 881-70-9 ]
[ 881-70-9 ]

-
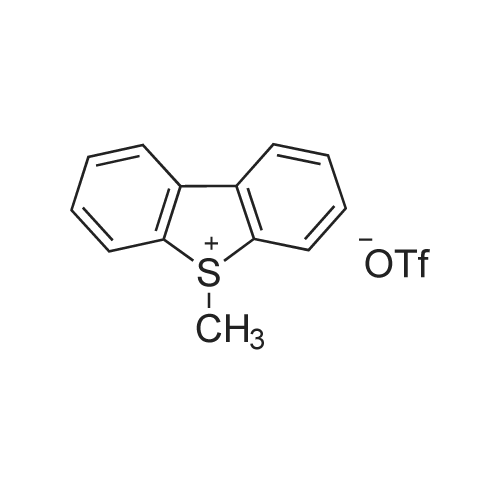 [ 112359-25-8 ]
[ 112359-25-8 ]

-
4’,5’-dimethoxy-2’-methylacetanilide
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 72% |
With palladium diacetate; copper(II) acetate monohydrate; trifluoroacetic acid In 1,2-dichloro-ethane at 50℃; for 16h; Sealed tube; |
|
- 16
-
 [ 1131-62-0 ]
[ 1131-62-0 ]

-
 [ 881-70-9 ]
[ 881-70-9 ]
| Yield | Reaction Conditions | Operation in experiment |
| 90% |
With zinc(II) chloride; hydroxylamine-O-sulfonic acid In water at 20℃; for 4h; |
Secondary Amides from Ketones; General Procedure
General procedure: To a stirring solution of ZnCl2 (0.05 mmol, 10 mol%) in H2O (2 mL) at r.t. in an open round-bottom flask, ketone 1 (0.5 mmol, 1.0 equiv) wasadded, followed by HOSA (1.5 equiv). The reaction mixture was stirred at the indicated temperature and for the duration indicated in Scheme 2. After completion, the reaction mixture was diluted with EtOAc (15 mL) and washed with sat. aq Na2CO3 (3 × 5 mL). The organic layer was washed with brine (5 mL) and dried over anhydrous Na2SO4.The crude product obtained after removal of all volatiles in vacuo was washed with n-hexane to remove some minor nonpolar impurities. |
| 89% |
With mesitylenesulfonylhydroxylamine In acetonitrile at 20℃; for 5h; |
General procedure for the preparation of amides from ketones
General procedure: To a round bottom flask, equipped with a magnetic stirring bar, was added ketone 1 (0.5 mmol, 1.0 equiv.) and acetonitrile (2 mL) at room temperature. To this stirred solution, freshly prepared O-(Mesitylsulfonyl)hydroxylamine 2 (2.0 equiv.) was added. The reaction mixture was stirred for the specified duration and temperature. The progress of the reaction was monitored by TLC. After completion, the reaction mixture was diluted with ethyl acetate (10 mL) and washed with a saturated aqueous NaHCO3 solution (3 x 5 mL). The combined organic layer was washed with brine solution and dried over anhydrous Na2SO4. Solvent was removed under reduced pressure to get the crude product. The reaction that required elevated temperature was stirred first at room temperature for 2 hours after addition of MSH and then heated at 70 °C for the specified time. |
| 80% |
With cesiumhydroxide monohydrate; copper(II) bis(trifluoromethanesulfonate); hydroxylamine-O-sulfonic acid In dichloromethane; 2,2,2-trifluoroethanol at 20℃; for 18h; |
Amides from Ketones
General procedure: To a stirred solution of Cu(OTf)2 (0.05 mmol, 10 mol%) in TFE/CH2Cl2 (1:4, 2-3 mL) were added ketone (0.5 mmol, 1.0 equiv), HOSA (2.0 equiv), and CsOH·H2O (2.0 equiv) at rt. The reaction mixture was maintained at the temperature and for the time indicated in Scheme 2. After completion, the mixture was diluted with CH2Cl2 (10 mL) and washed with sat. aq Na2CO3 (3 × 5 mL). The combined organic layers were washed with brine (5 mL) and dried (anhyd Na2SO4). The crude product obtained after removal of all volatiles in vacuo was purified by SiO2 (100-200 mesh) chromatography using EtOAc/hexane as eluent. |
|
Multi-step reaction with 2 steps
1.1: hydroxylamine hydrochloride; sodium acetate / ethanol; water / 80 °C
2.1: triethylamine; fluorosulfonyl fluoride / acetonitrile / 20 °C
2.2: 20 °C |
|

Reference:
[1]Verma, Saumya; Kumar, Puneet; Khatana, Anil K.; Chandra, Dinesh; Yadav, Ajay K.; Tiwari, Bhoopendra; Jat, Jawahar L.
[Synthesis, 2020, vol. 52, # 21, p. 3272 - 3276]
[2]Chandra, Dinesh; Verma, Saumya; Pandey, Chandra Bhan; Yadav, Ajay K.; Kumar, Puneet; Tiwari, Bhoopendra; Jat, Jawahar L.
[Tetrahedron Letters, 2020, vol. 61, # 18]
[3]Anugu, Raghunath Reddy; Chandra, Dinesh; Falck, John R.; Jat, Jawahar L.; Munnuri, Sailu; Verma, Saumya
[Synthesis, 2019, vol. 51, # 19, p. 3709 - 3714]
[4]Zhang, Guofu; Zhao, Yiyong; Xuan, Lidi; Ding, Chengrong
[European Journal of Organic Chemistry, 2019, vol. 2019, # 30, p. 4911 - 4915]

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping


















