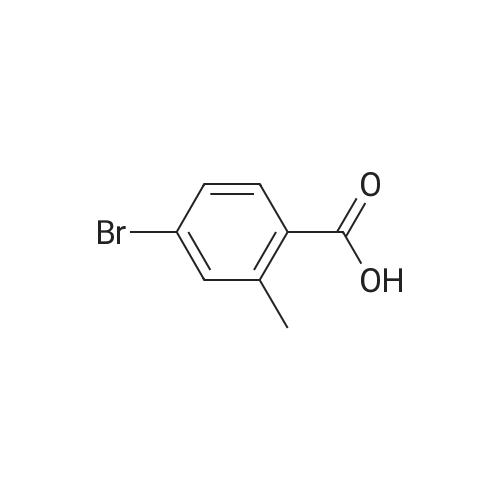
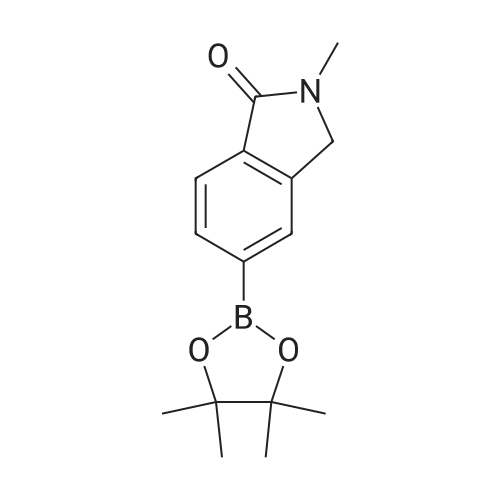
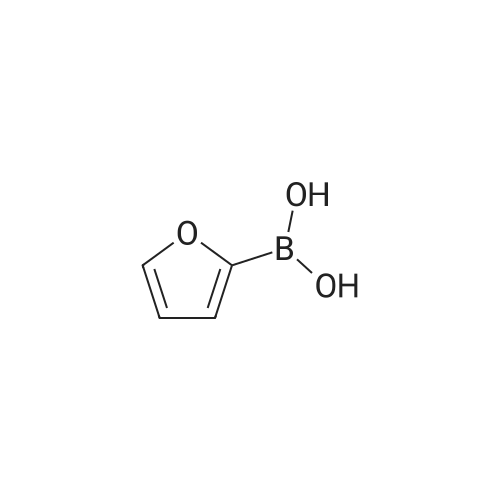
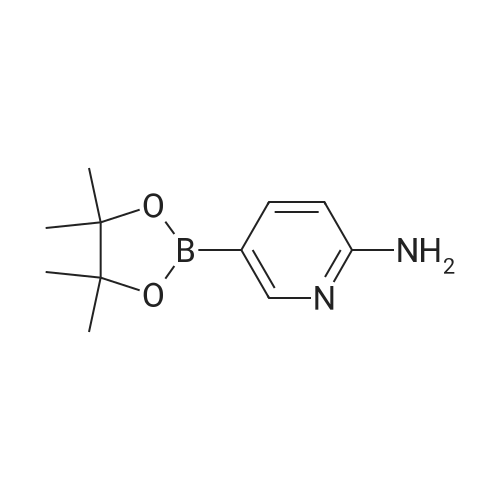
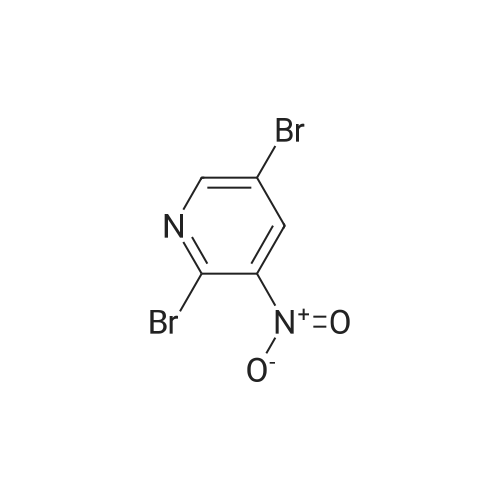
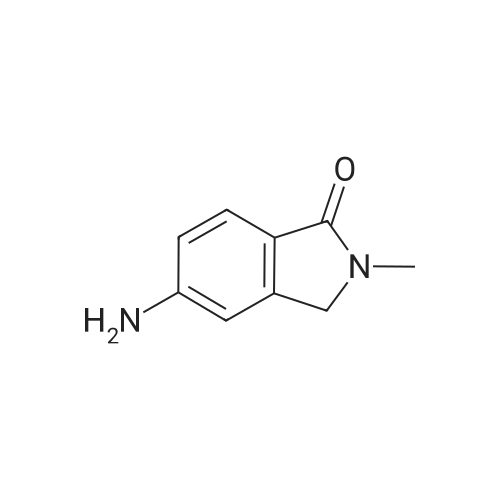
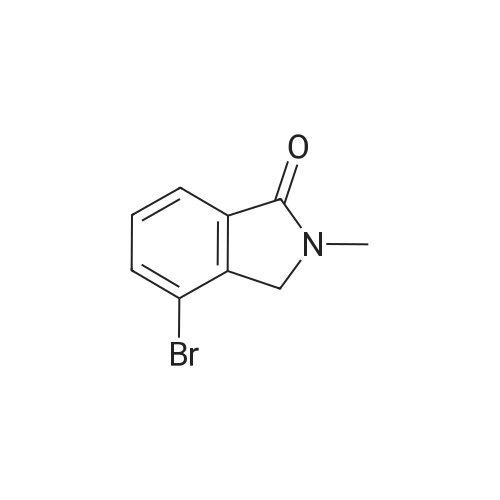
| 89% |
With dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; potassium acetate; In 1,4-dioxane; at 85℃;Inert atmosphere; |
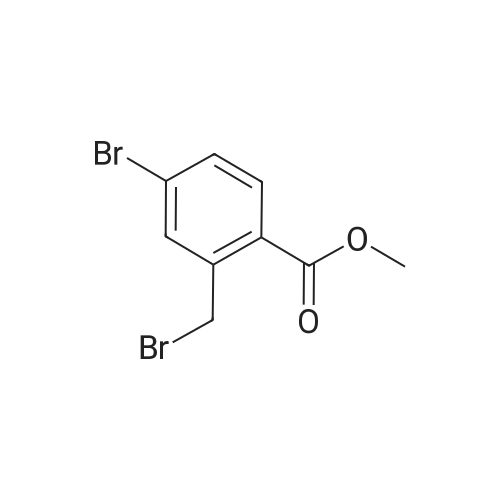
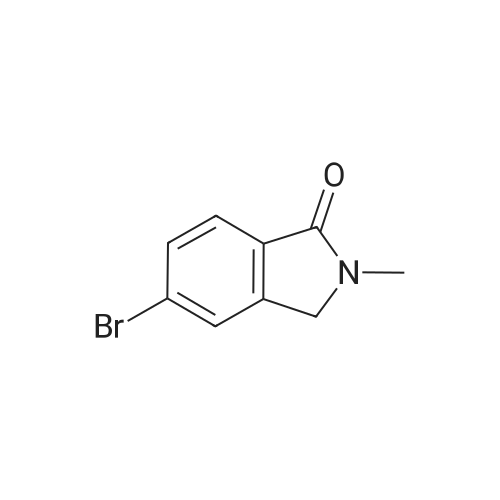
[00532] Intermediate Sic: 2-methyl-5-(4,4,5,5-tetramethyl-1 ,3,2-dioxaborolan-2-yI)isoindolin-1-one[00533] A round bottomed flask containing bis(pinacolato)diboron (354mg, 1 .3gmmol) and KOAc (326mg, 3.32mmol) in 1 ,4-dioxane (6.5mL) was evacuated/backfilled with nitrogen. Pd(dppt)C12.CH2CI2 (54mg, 0.O7mmol) was added and the flask was evacuated/backfilled with nitrogen again. The reaction mixture was then stirred and heated at 85 C overnight. After this timethe mixture was cooled to room temperature, filtered through a plug of celite and the solid was washed with EtOAc. The filtrate was concentrated and the residue purified by column chromatography using an eluent of 0-100% EtOAc in heptane to give 2-methyl-5-(4,4,5,5- tetramethyl-1,3,2-dioxaborolan-2-yl)isoindolin-1-one (324mg,1.lgmmol, 89% yield) as a brown solid.1H NMR (CDCI3, 400MHz) O/ppm: 7.92 (1H, dd, J= 7.6Hz, 0.4Hz), 7.90-7.89 (1H, m), 7.85 (1H, dd, J 7.6Hz, 0.4Hz), 4.39 (2H, 5), 3.23 (3H, 5), 1.38 (12H, 5).MS Method 3: RT: 3.61 mi m/z 274.1 [M+H] |
|
With potassium acetate; XPhos;tris-(dibenzylideneacetone)dipalladium(0); In 1,4-dioxane; at 85℃; for 22h; |
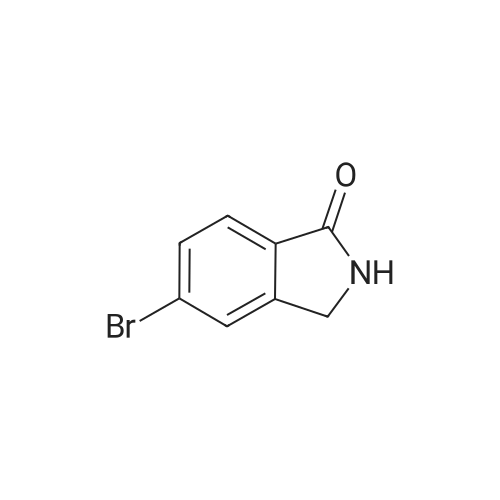
In a sealable tube was combined Pd2dba3 (0.0270 g, 0.0295 mmol), 2- (dicyclohexylphosphino) -2' , 4', 6'- tri-i-propyl-1, 1' -biphenyl (0.0563 g, 0.118 mmol) , 4 , 4, 5, 5- tetramethyl-2- (4,4, 5 , 5-tetramethyl-l, 3 , 2-dioxaborolan-2-yl) - 1, 3, 2-dioxaborolane (0.450 g, 1.77 mmol), 5-bromo-2- <n="113"/>methylisoindolin-1-one (see Tsuritani, T., et al Synlett 2006, 5 801-803} (0.267 g, 1.18 mmol) , potassium acetate (0.232 g, 2.36 mmol) and 2 mL dioxane . The mixture was blanketed with N2, sealed and heated at 80 C for 22 h. The mixture was allowed to cool to rt then diluted with EtOAc, and the organic layer washed with water, sat. NaHCO3, then dried over Na2SO4, filtered and evaporated. The residue was purified via flash chromatography using a EtOAc in CH2Cl2 gradient. The title compound was collected as a tan solid. |
|
With potassium acetate;(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; In 1,4-dioxane; at 100℃; for 4h;Inert atmosphere of nitrogen; |
To a solution of 5-bromo-2-methylisoindolin-l-one (Example 31, step (i), 60.4 g) in dioxane (2 L) was added 4,4,4',4',5,5,5',5'-octamethyl-2,2'-bi(l,3,2-dioxaborolane) (67.8 g), potassium acetate (65.4 g) and Pd(dppf)Cl2 (6 g) under an atmosphere of nitrogen. The mixture was heated to 1000C and stirred for 4 h. The reaction mixture was cooled to room temperature and filtered. The filtrate was concentrated and the crude product was purified by chromatography on silica eluting with petroleum ether / ethyl acetate (6:1 to 2: 1) to afford the sub-titled compound (72 g).1H NMR (400 MHz, CDCl3): delta 7.89-7.88 (m, 2H), 7.83-7.81 (m, IH), 4.35 (s, 2H), 3.19 (s, 3H), 10.34 (s, 12H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping