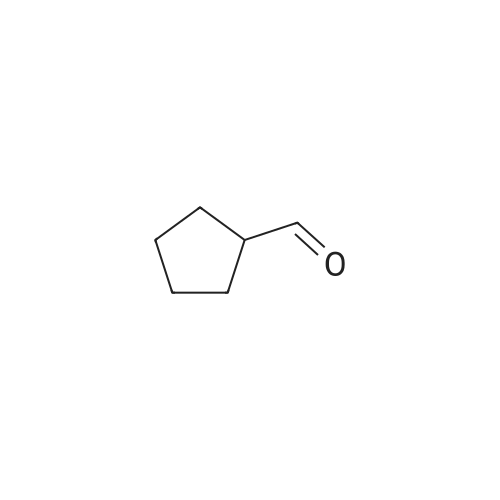
|
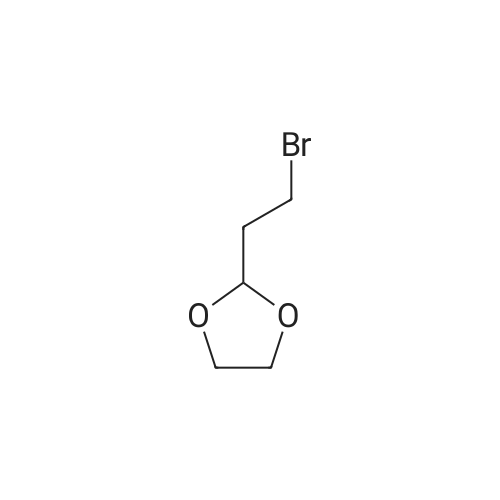
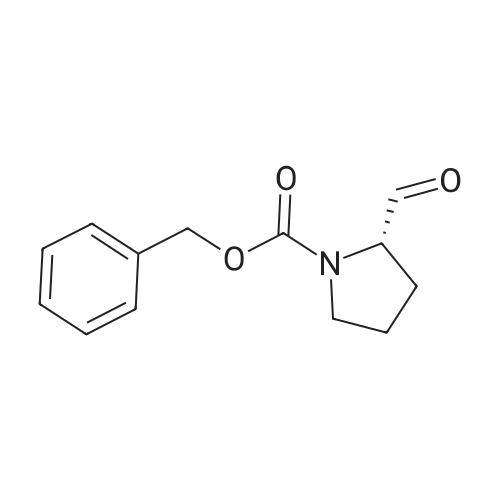
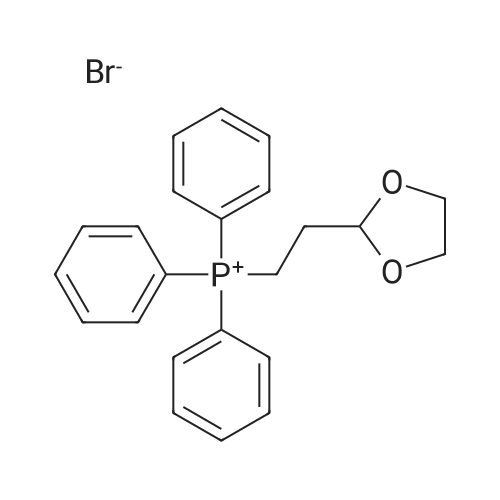
Stage #1: 2-(1,3-dioxolan-2-yl)ethyl(triphenyl)phosphonium bromide With potassium <i>tert</i>-butylate In tetrahydrofuran at 20℃; for 1.5h;
Stage #2: (S)-2-formylpyrrolidine-1-carboxylic acid benzyl ester In tetrahydrofuran at 20 - 50℃; for 4h; |
55
Reference Example 55; L(-)-proline (17.0 g) was dissolved in tetrahydrofuran (350 ml) and a 1 N aqueous sodium hydroxide solution (296 ml), and then benzyl chlorocarbonate (23.2 ml) was added dropwise thereto at 0°C under a nitrogen atmosphere. The resulting mixture was returned to room temperature and stirred overnight. 1 N Hydrochloric acid (296 ml) was added thereto at 0°C, and the mixture was extracted with ethyl acetate. The organic layer was washed with saturated brine, and dried over magnesium sulfate. The solvent was distilled off under reduced pressure to give 1-[(benzyloxy)carbonyl]-L-proline, which was as such dissolved in tetrahydrofuran (250 ml), and then triethylamine (26.8 ml) was added thereto. To the resulting solution was added dropwise ethyl chlorocarbonate (16.9 ml) at 0°C under a nitrogen atmosphere. The mixture was returned to room temperature and stirred for 1 hour, and the insolubles were removed by filtration. To the filtrate was added dropwise an aqueous sodium borohydride (11.2 g) solution (100 ml) at 0°C, and the mixture was returned to room temperature and stirred for 3 hours under a nitrogen atmosphere. Next, water was added thereto, and the mixture was extracted with ethyl acetate twice. The organic layer was washed with saturated brine. The solvent was distilled off under reduced pressure, and the resulting residue was purified by silica gel column chromatography (hexane : ethyl acetate 2 : 1 → 1 : 4) to give benzyl (2S)-2-(hydroxymethyl)pyrrolidine-1-carboxylate (25.0 g) as a colorless oily material. To a solution of oxalyl chloride (6.23 ml) in dichloromethane (70 ml) was added dropwise a solution of dimethyl sulfoxide (10.9 ml) in dichloromethane (100 ml) at -78°C under an argon atmosphere. The mixture was stirred as such for 15 minutes, and then a solution of benzyl (2S)-2-(hydroxymethyl)pyrrolidine-1-carboxylate (12.0 g) in dichloromethane (80 ml) was added dropwise thereto. The mixture was stirred as such for 15 minutes, and then triethylamine (42.7 ml) was added dropwise thereto, followed by stirring as such for 20 minutes. The resulting mixture was returned to room temperature and then water was added thereto, followed by separation. The organic layer was washed with 1 N hydrochloric acid twice, an aqueous 4% sodium carbonate solution and saturated brine, respectively, and then dried over magnesium sulfate. The solvent was distilled off under reduced pressure, and the resulting residue was purified by silica gel column chromatography (hexane : ethyl acetate = 2 : 1 → 1 : 1) to give benzyl (2S)-2-formylpyrrolidine-1-carboxylate. To [2-(1,3-dioxolan-2-yl)ethyl]triphenylphosphonium bromide (90.4 g) was added tetrahydrofuran (400 ml), further added t-butoxypotassium, and the mixture was stirred at room temperature for 1.5 hours under a nitrogen atmosphere. To the resulting mixture was added dropwise a solution of benzyl (2S)-2-formylpyrrolidine-1-carboxylate in tetrahydrofuran (200 ml), and the mixture was stirred at room temperature for 1 hour and at 50°C for 3 hours under a nitrogen atmosphere. After returning to room temperature, water was added thereto and the mixture was extracted with ethyl acetate. The organic layer was washed with saturated brine, and dried over magnesium sulfate. The solvent was distilled off under reduced pressure, and the resulting residue was purified by silica gel column chromatography (hexane : ethyl acetate = 3 : 1 → 2 : 1) to give benzyl (2S)-2-[3-(1,3-dioxolan-2-yl)-1-propenyl]pyrrolidine-1-carboxylate (15.1 g). Benzyl (2S)-2-[3-(1,3-dioxolan-2-yl)-1-propenyl]pyrrolidine-1-carboxylate (15.0 g) was dissolved in ethanol (200 ml), and then 10% palladium carbon (4.0 g, water content: 50%) was added thereto, followed by overnight stirring at room temperature under a hydrogen atmosphere. The insolubles were removed by filtration, and then the solvent was distilled off under reduced pressure. The resulting residue was dissolved in tetrahydrofuran (200 ml) and a 1 N aqueous sodium hydroxide solution (150 ml), and then benzyl chlorocarbonate (10.1 ml) was added dropwise thereto at 0°C under a nitrogen atmosphere. The mixture was returned to room temperature, stirred for 3 hours and extracted with ethyl acetate. The organic layer was washed with saturated brine, and dried over magnesium sulfate. The solvent was distilled off under reduced pressure, and the resulting residue was purified by silica gel column chromatography (hexane : ethyl acetate = 7 : 1) to give benzyl (2R)-2-[3-(1,3-dioxolan-2-yl)propyl]pyrrolidine-1-carboxylate (13.6 g). Benzyl (2R)-2-[3-(1,3-dioxolan-2-yl)propyl]pyrrolidine-1-carboxylate (13.0 g) was dissolved in tetrahydrofuran (130 ml), and then 2 N hydrochloric acid (305 ml) was added thereto at 0°C. After stirring the resulting mixture at room temperature for 3.5 hours, a 1 N aqueous sodium hydroxide solution (610 ml) was added thereto at 0°C. The mixture was extracted with ethyl acetate, the organic layer was then washed with saturated brine, and dried over magnesium sulfate. The solvent was distilled off under reduced pressure, and the resulting residue was purified by silica gel column chromatography (hexane : ethyl acetate = 2 : 1) to give colorless benzyl (2R)-2-(4-oxobutyl)pyrrolidine-1-carboxylate (8.4 g). To a solution of benzyl (2R)-2-(4-oxobutyl)pyrrolidine-1-carboxylate (8.0 g) and 1-methyl-1-cyclohexene (45.9 ml) in tert-butanol (250 ml) was added dropwise an aqueous solution (200 ml) of sodium chlorite (36.8 g) and sodium dihydrogen phosphate dihydrate (40.9 g) at room temperature. The mixture was stirred as such for 3 hours, and then 1 N hydrochloric acid (40 ml) was added thereto at 0°C. The mixture was extracted with ethyl acetate, washed with an aqueous sodium thiosulfate solution twice and saturated brine once, and then dried over magnesium sulfate. The solvent was distilled off under reduced pressure, and the resulting residue was purified by silica gel column chromatography (hexane : ethyl acetate = 3 : 1 → methanol : ethyl acetate = 1 : 19). The resulting residue was dissolved in ethanol (200 ml), 10% palladium carbon (3.0 g, water content: 50%) was added thereto, and the mixture was stirred for 6 hours under a hydrogen atmosphere. The insolubles were removed by filtration, and then the solvent was distilled off under reduced pressure. The resulting residue was washed with hexane-ethyl acetate to give 4-[(2R)-pyrrolidin-2-yl]butanoic acid (2.63 g) as colorless crystals. 4-[(2R)-pyrrolidin-2-yl]butanoic acid (1.21 g), 5-bromo-2-chloronicotinaldehyde (1.3 g) and sodium carbonate (1.63 g) were added to dimethyl sulfoxide (30 ml) and water (15 ml), and then the mixture was stirred at 90°C for 4.5 hours. After cooling to 0°C, water was added thereto, 1 N hydrochloric acid (30 ml) was then added thereto, and the mixture was extracted with ethyl acetate. The organic layer was washed with water and saturated brine, and dried over magnesium sulfate. The solvent was distilled off under reduced pressure, and the resulting residue was as such dissolved in N,N-dimethylformamide (20 ml), and then potassium carbonate (2.45 g) and iodomethane (1.1 ml) were added thereto, followed by stirring at room temperature for 1 hour under a nitrogen atmosphere. Water was added thereto and the mixture was then extracted with ethyl acetate. The organic layer was washed with water and saturated brine, and dried over magnesium sulfate. The solvent was distilled off under reduced pressure, and the resulting residue was purified by silica gel column chromatography (hexane : ethyl acetate = 16 : 1 → hexane : ethyl acetate = 4 : 1) to give methyl 4-[(2R)-1-(5-bromo-3-formylpyridin-2-yl)pyrrolidin-2-yl]butanoate (1.94 g) as a yellow oily material. To a solution of methyl 4-[(2R)-1-(5-bromo-3-formylpyridin-2-yl)pyrrolidin-2-yl]butanoate (1.9 g) in dimethyl carbonate (50 ml) was added sodium methoxide (28% solution in methanol, 2.06 g), and the mixture was heated at 70°C for 3 hours under a nitrogen atmosphere. After ice-cooling the resulting mixture, water was added thereto and the mixture was extracted with ethyl acetate. The organic layer was washed with saturated brine, and dried over magnesium sulfate. The solvent was distilled off under reduced pressure, and the resulting residue was purified by silica gel column chromatography (hexane : ethyl acetate = 9 : 1 → 4 : 1). The resulting residue was washed with hexane-ethyl acetate to give methyl (8aR)-3-bromo-7,8,8a,9,10,11-hexahydropyrido[3,2-g]pyrrolo[1,2-a]azocine-6-carboxylate (1.33 g) as yellow crystals. A suspension of methyl (8aR)-3-bromo-7,8,8a,9,10,11-hexahydropyrido[3,2-g]pyrrolo[1,2-a]azocine-6-carboxylate (1.2 g), 4-(2-butoxyethoxy)phenylboric acid (1.10 g) and potassium carbonate (1.28 g) in toluene (20 ml), ethanol (2 ml) and water (2 ml) was stirred for 30 minutes under an argon atmosphere. Tetrakis(triphenylphosphine) palladium (206 mg) was added thereto, and the mixture was heated at 115°C for 6 hours under an argon atmosphere and was allowed to cool. Water was added thereto and the mixture was extracted with ethyl acetate. The organic layer was washed with saturated brine, and dried over magnesium sulfate. The solvent was distilled off under reduced pressure, and the resulting residue was purified by silica gel column chromatography (hexane : ethyl acetate = 19 : 1 → hexane : ethyl acetate = 4 : 1) to give methyl (8aR)-3-[4-(2-butoxyethoxy)phenyl]-7,8,8a,9,10,11-hexahydropyrido[3,2-g]pyrrolo[1,2-a]azocine-6-carboxylate (700 mg) as a yellow oily material. To a solution of methyl (8aR)-3-[4-(2-butoxyethoxy)phenyl]-7,8,8a,9,10,11-hexahydropyrido[3,2-g]pyrrolo[1,2-a]azocine-6-carboxylate (690 mg) in THF (25 ml) and methanol (25 ml) was added a 1 N aqueous sodium hydroxide solution (6.1 ml), and the mixture was heated at 90°C for 3 hours. After cooling the resulting mixture to 0°C, water was added thereto and the mixture was neutralized with 1 N hydrochloric acid, and then extracted with ethyl acetate. The organic layer was washed with saturated brine, and dried over magnesium sulfate. The solvent was distilled off under reduced pressure, and the resulting residue was recrystallized from ethyl acetate to give (8aR)-3-[4-(2-butoxyethoxy)phenyl]-7,8,8a,9,10,11-hexahydropyrido[3,2-g]pyrrolo[1,2-a]azocine-6-carboxylic acid (403 mg) as yellow crystals. m.p. 215.5-216.5°C Elementary analysis C26H32N2O4, Calcd. C, 71.53 ; H, 7.39 ; N, 6.42 ; Found. C, 71.43 ; H, 7.48 ; N, 6.19. [α]D = +196.3° (C = 0.404%, in chloroform) |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping