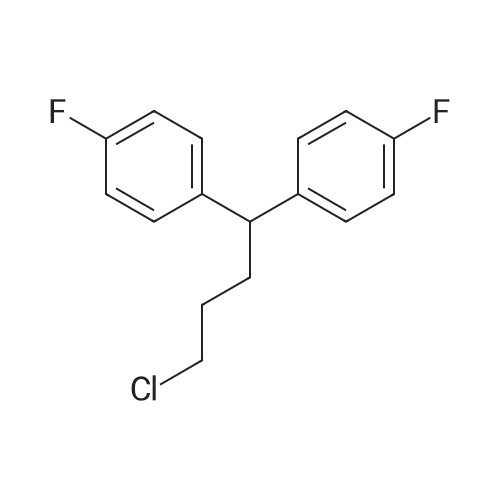
| 1.7 g (17%) |
In N-methyl-acetamide; ethyl acetate; |
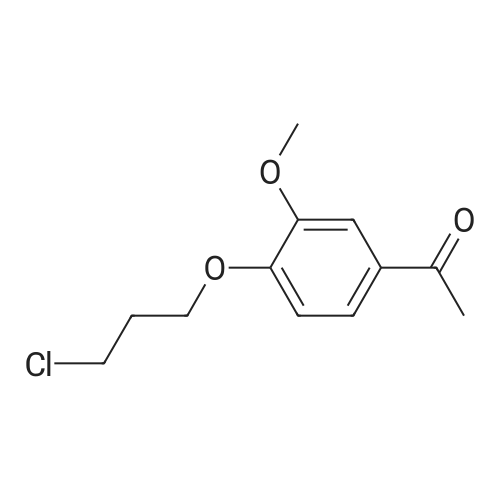
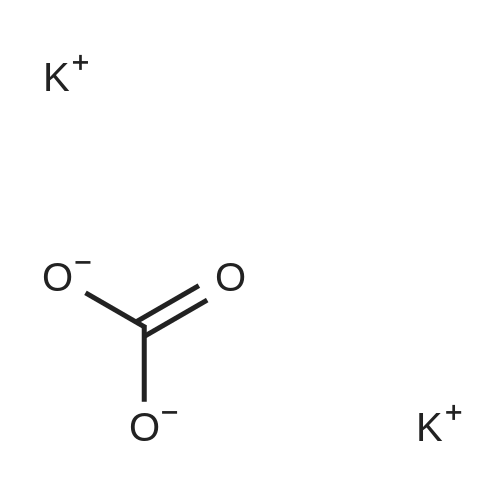
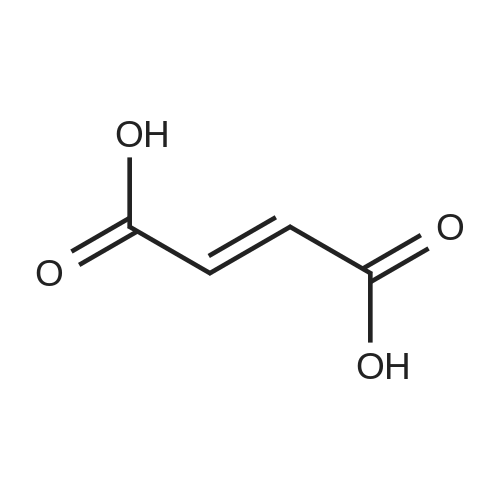
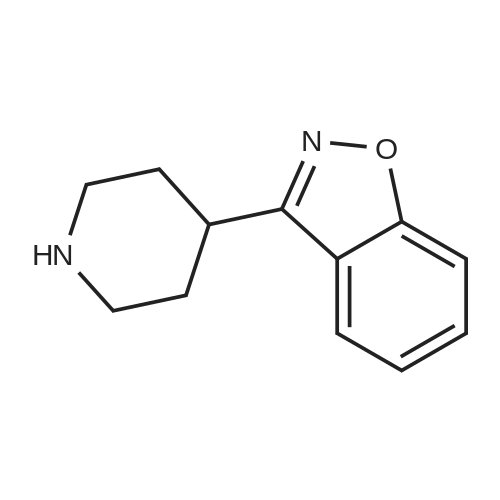
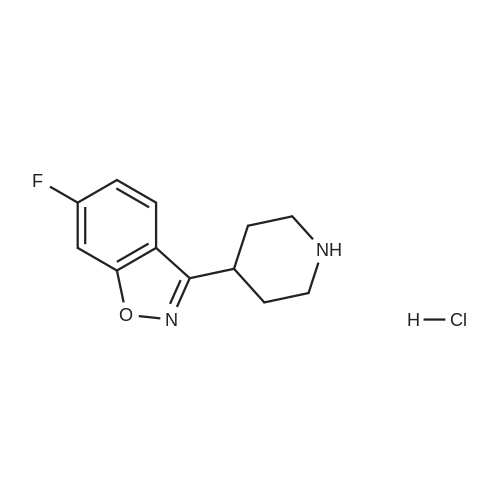
EXAMPLE 6 1-[4-[2-[4-(1,2-Benzisoxazol-3-yl)-1-piperidinyl]ethoxy]-3-methoxyphenyl]ethanone fumarate A mixture of <strong>[84163-22-4]3-(4-piperidinyl)-1,2-benzisoxazole hydrochloride</strong> (4.8 g, 0.02 mol), K2 CO3 (5.2 g, 0.04 mol), 1-[4-(2-chloroethoxy)-3-methoxyphenyl]ethanone (5.0 g, 0.022 mol), and dimethylformamide (90 ml) was heated at 90 C. for 16 hours. The reaction was poured into water and the aqueous mixture was extracted with ethyl acetate. The ethyl acetate was washed (water), dried (MgSO4), and the solvent was concentrated to afford an oil. Upon standing, the oil solidified to afford a beige solid. The crude solid was recrystallized twice from ethyl alcohol to afford 5.9 g of an off-white solid. The solid was dissolved in ethyl acetate, and fumaric acid (1.2 g, 1.1 equiv.) was added. The mixture was heated briefly on a steam bath, and then stirred at ambient temperature for 2 hours. An initial green oil settled out and the supernatant solution was decanted. Ether was added to the decantate and 4.0 g of a white fumarate salt was collected. The salt was recrystallized twice from ethanol-ether to yield 1.7 g (17%) of 1-[4-[2-[4-(1,2-benzisoxazol-3-yl)-1-piperidinyl]ethoxy]-3-methoxyphenyl]ethanone fumarate, m.p.=127-129 C. |
| 1.7 g (17%) |
In N-methyl-acetamide; ethyl acetate; |
EXAMPLE 6 1-[4-[2-[4-(1,2-Benzisoxazol-3-yl)-1-piperidinyl]ethoxy]-3-methoxyphenyl]ethanone fumarate A mixture of <strong>[84163-22-4]3-(4-piperidinyl)-1,2-benzisoxazole hydrochloride</strong> (4.8 g, 20 mmol), K2 CO3 (5.2 g, 40 mmol), 1-[4-(2-chloroethoxy)-3-methoxyphenyl]ethanone (5.0 g, 22 mmol), and dimethylformamide (90 ml) was heated at 90 C. for 16 hours. The reaction was poured into water and the aqueous mixture was extracted with ethyl acetate. The ethyl acetate was washed (water), dried (MgSO4), and the solvent was concentrated to afford an oil. Upon standing, the oil solidified to afford a beige solid. The crude solid was recrystallized twice from ethyl alcohol to afford 5.9 g of an off-white solid. The solid was dissolved in ethyl acetate, and fumaric acid (1.2 g, 1.1 equiv.) was added. The mixture was heated briefly on a steam bath, and then stirred at ambient temperature for 2 hours. An initial green oil settled out and the supernatant solution was decanted. Ether was added to the decantate and 4.0 g of a white fumarate salt was collected. The salt was recrystallized twice from ethanol-ether to yield 1.7 g (17%) of 1-[4-[2-[4-(1,2-benzisoxazol-3-yl)-1-piperidinyl]ethoxy]-3-methoxyphenyl]ethanone fumarate, m.p.=127-129 C. ANALYSIS: Calculated for C23 H26 N2 O4.C4 H4 O4: 63.52%C, 5.92%H, 5.49%N; Found: 63.00%C, 5.87%H, 5.42%N. |
| 1.7 g (17%) |
In N-methyl-acetamide; ethyl acetate; |
EXAMPLE 6 1-[4-[2-[4-(1,2-Benzisoxazol-3-yl)-1-piperidinyl]ethoxy]-3-methoxy-phenyl]ethanone fumarate A mixture of <strong>[84163-22-4]3-(4-piperidinyl)-1,2-benzisoxazole hydrochloride</strong> (4.8 g, 20 mmol), K2 CO3 (5.2 g, 40 mmol), 1-[4-(2-chloroethoxy)-3-methoxyphenyl]ethanone (5.0 g, 22 mmol), and dimethylformamide (90 ml) was heated at 90 C. for 16 hours. The reaction was poured into water and the aqueous mixture was extracted with ethyl acetate. The ethyl acetate was washed (water), dried (MgSO4), and the solvent was concentrated to afford an oil. Upon standing, the oil solidified to afford a beige solid. The crude solid was recrystallized twice from ethyl alcohol to afford 5.9 g of an off-white solid. The solid was dissolved in ethyl acetate, and fumaric acid (1.2 g, 1.1 equiv.) was added. The mixture was heated briefly on a steam bath, and then stirred at ambient temperature for 2 hours. An initial green oil settled out and the supernatant solution was decanted. Ether was added to the decantate and 4.0 g of a white fumarate salt was collected. The salt was recrystallized twice from ethanol-ether to yield 1.7 g (17%) of 1-[4-[2-[4-(1,2-benzisoxazol-3-yl)-1-piperidinyl]ethoxy]-3-methoxyphenyl]ethanone fumarate, m.p.=127-129 C. ANALYSIS: Calculated for C23 H26 N2 O4: 63.52%C 5.92%H 5.49%N; Found: 63.00%C 5.87%H 5.42%N. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping