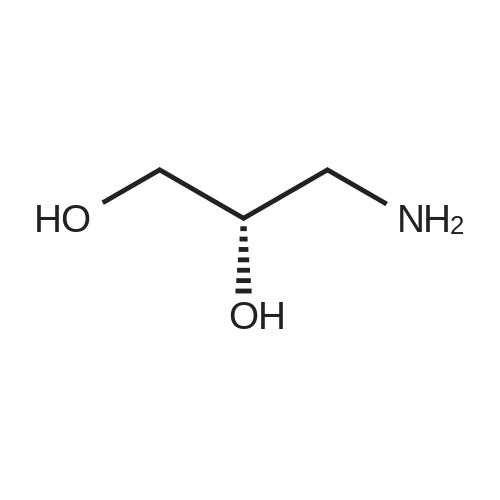
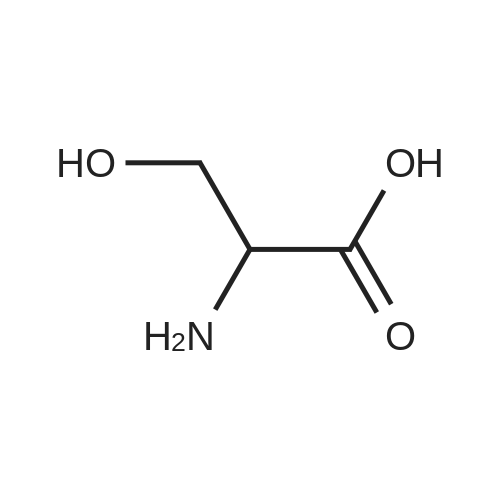
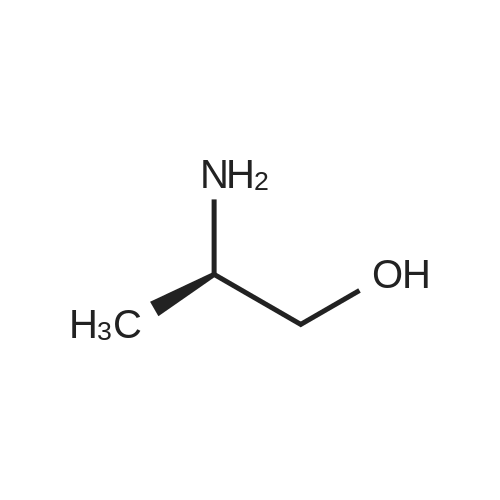
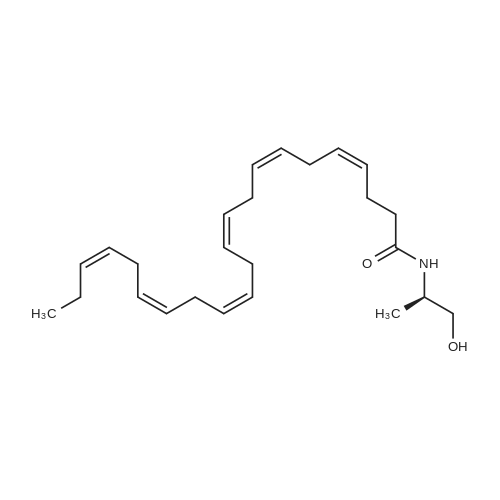
|
With lithium hydroxide;Inert atmosphere; Darkness; |
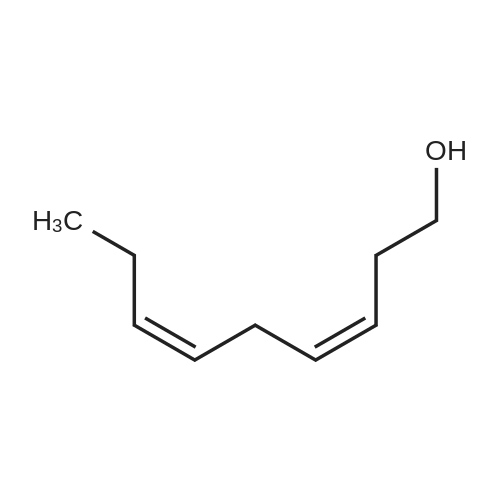
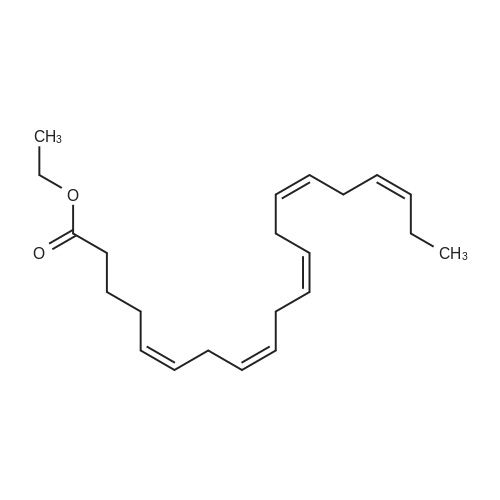
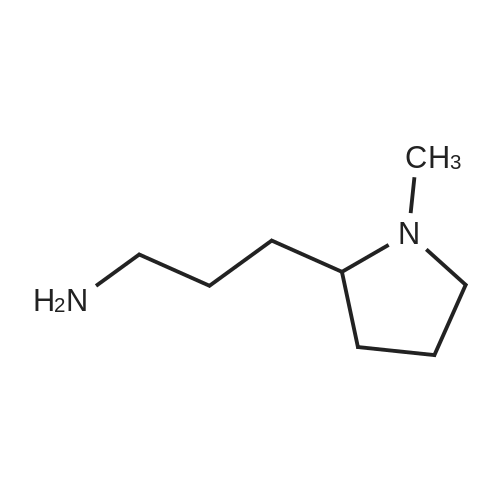
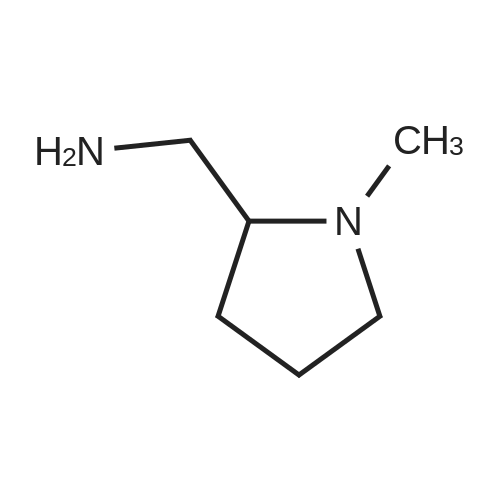
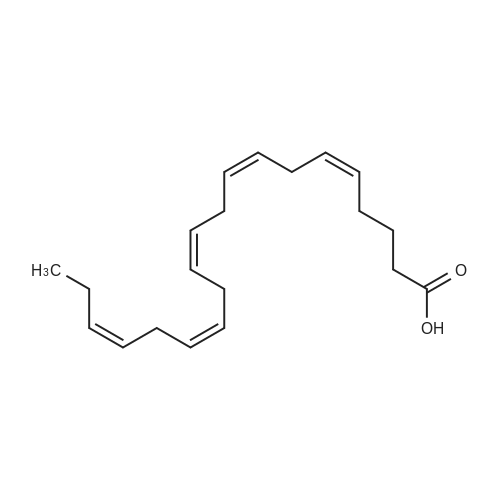
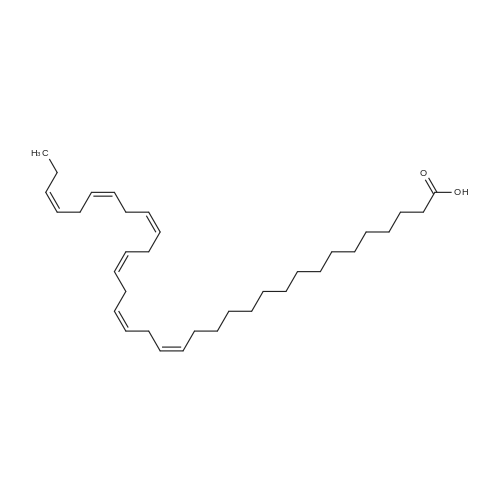
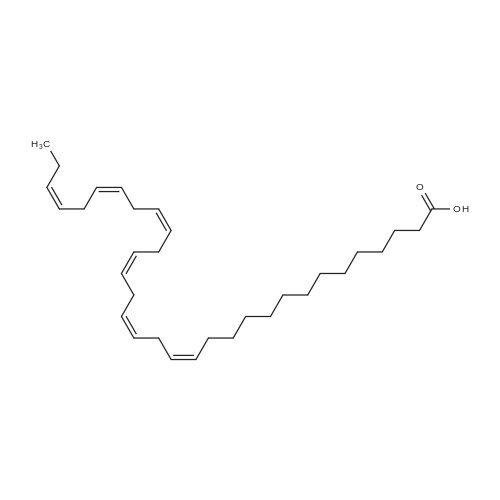
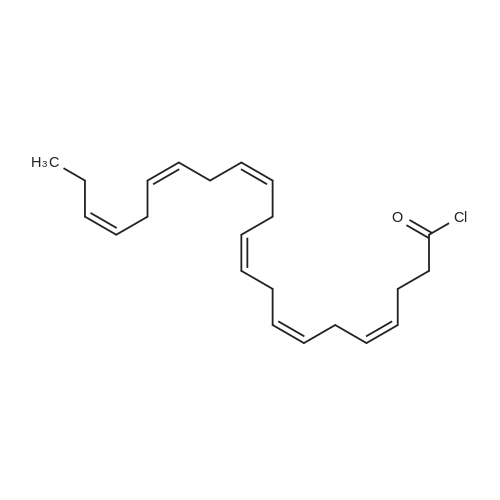
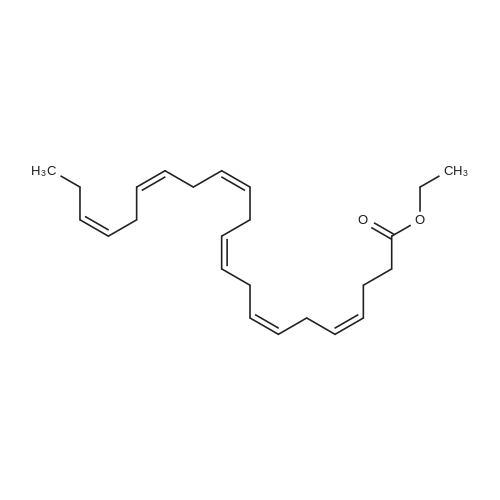
Example 1 : Overview of l, l, l-trifluoro-3-(((2E,6Z,9Z, 12Z, 15Z,)-octadeca- 2,6,9.1 2, 1 -pcntaein- l -yl )tliio)propan-2-one synthesis The following information provides a general overview of the synthesis of the polyunsaturated ketone. Referring to Scheme 1, Step A and Step B are mentioned in more detail in Example 6, below. All steps toward making the C18 allylic alcohol (Scheme 1, below) were carried out essentially as described in J. Chem. Sec, Perkin Trans. 1, 2000, 2271 - 2276 ( Skattebol and Holmeide), with one modification: instead of sodium borohydride in the reduction of aldehyde to alcohol, d i i sobu ty I a I u m i n i u m hydride (DIBAH) was used to reduce over-reduction of the trans double bond. During the three last steps ( Scheme 1 , below), the following precautions modifications were introduced: All reactions were carried out under an argon atmosphere. The lights in the fume hoods were to be turned off. No other precautions regarding light protection were considered necessary. In addition, the 3 last steps (called A, B and C, respectively) are performed in the presence of alpha-tocopherol. The sequence of reagent addition in the Mitsonobu reaction was considered to be important in order to avoid isomerization. It is anticipated that it is of crucial importance that all of the thioacctic acid has reacted completely prior to introduction of C 18 allyl ic alcohol. Furthermore, both the crude and purified product of step (A) are flushed with argon capped with a septum and placed in the freezer when stored. Step (B) is carried out without purification, but again: the crude product is to be stored at -18C under argon atmosphere if storage is necessary. During the last step great care was taken to ensure that both the crude product and purified material was kept under argon. ethyl (4Z,7Z, 10Z, 13Z, 16Z, 19Z)-docosa-4,7, 10,13,16,19- (4Z,7Z, 10Z, 13 Z, 16Z, 19Z)-docosa- hexaenoate 4,7,10,13, 16,19-hexaenoic acid 1) KI, I2, KHC03 THF, H20, 3 days 2) MeOH, K2C03 (3Z,6Z,9Z,12Z,15-octadeca-3,6,9,12,15- 1} HCOOH ( 1 1}> A0 (150 ml) 2) LiOH, H20-MeOH, 0 oC methyl 3-(3-((2Z,5Z,8Z,l lZ,14Z)-heptadeca- 2,5,8,11.14-pentaen- 1 -yl)oxiran-2-yl)propanoate 3) NaI04 MeOH-H20 DBU Et,0 entaen- 1 -ol (2£,6Z,9Z,12Z,15Z)-octadeca-2,6,9, 12, 15-pentaenal C18 allylic iilc hol STEP A DIAD, PPh3 Mitsonobu CH3COSH (2EAZ,9Z, 12Z, 15Z)-octadeca-2,6,9, 12,15- pentaene-1 -thiol MeOH S-((2E,6Z,9Z, 12Z, 15Z)-octadeca-2,6,9, 12, 15-pentaen-l-yl) ethanethioate aHC03 1,1,1 -trifluoro-3 -(((2i?,6Z,9Z, 12Z, 15 Z)-octadeca- 2,6,9, 12 , 15-pentaen- 1 -yl)thio)propan-2-one Scheme 1 : Overview of synthesis of l, l, l-trifluoro-3-(((2E,6Z,9Z, 12Z, 15Z,)- octadeca-2,6,9, 12, 15-pentaein- l-yl)thio)propan-2-one |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping