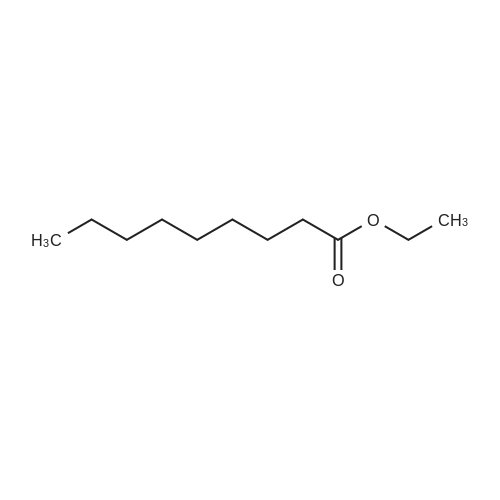
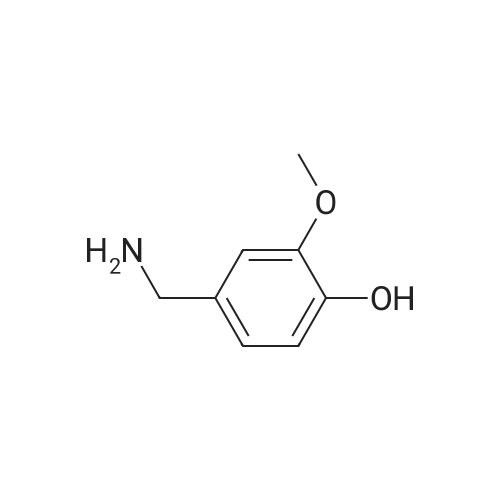
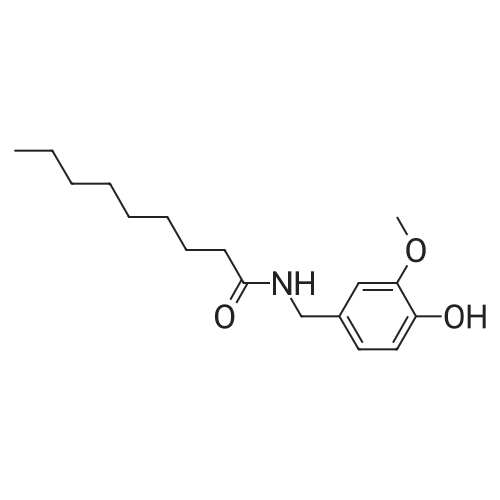
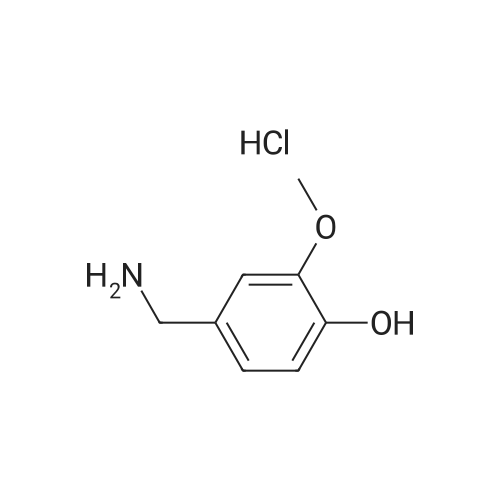
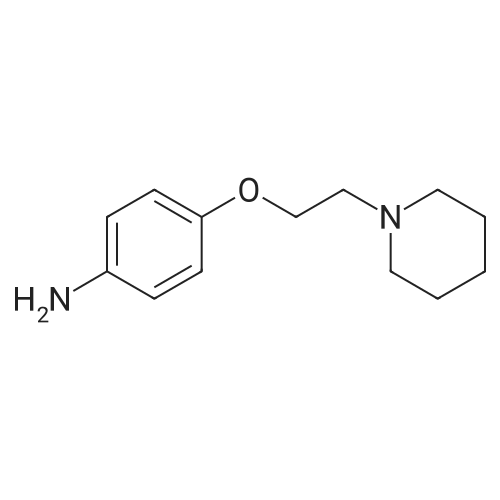
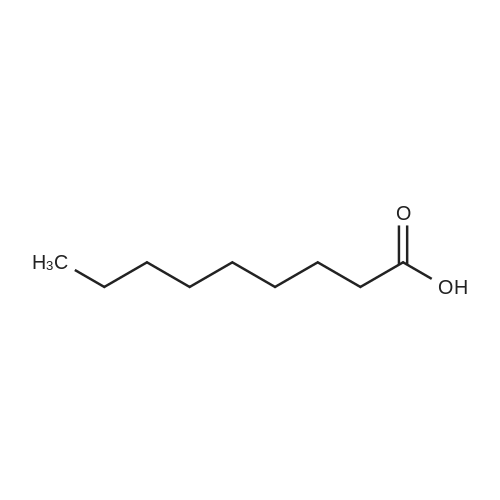
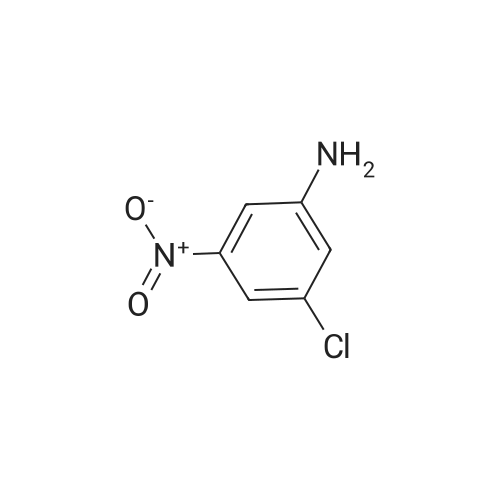
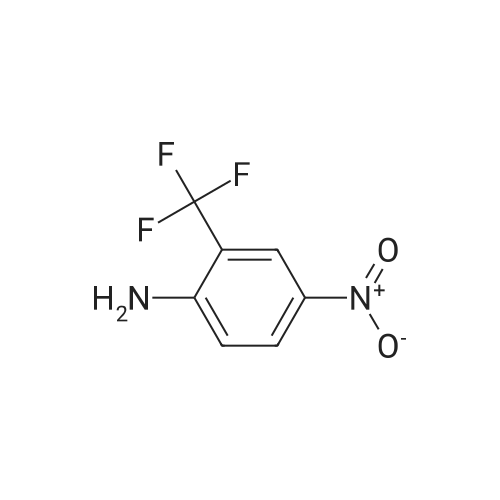
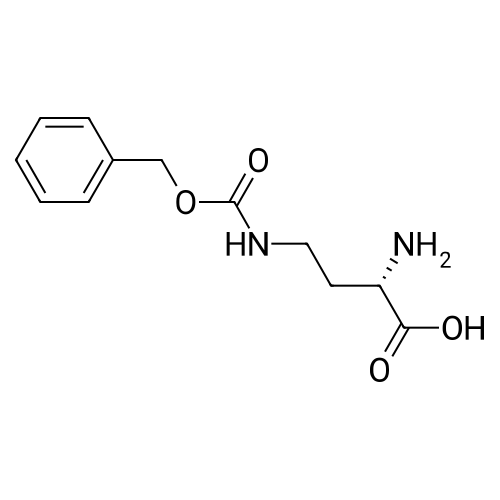
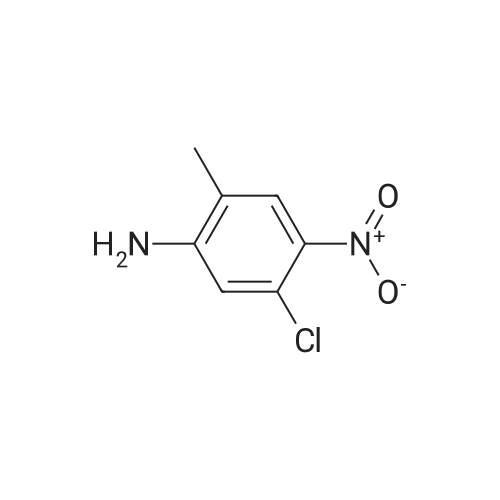
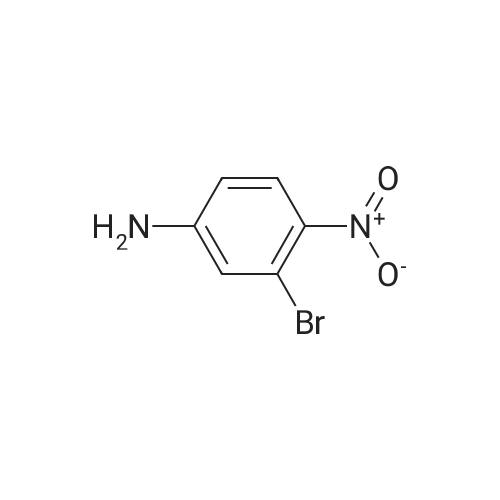
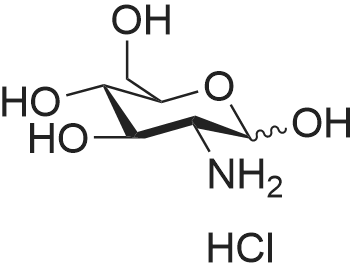
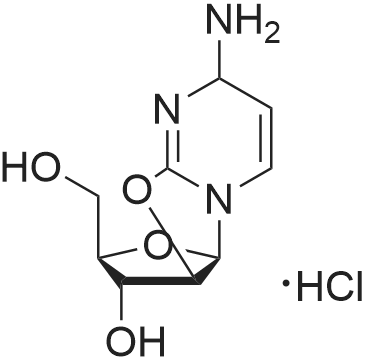
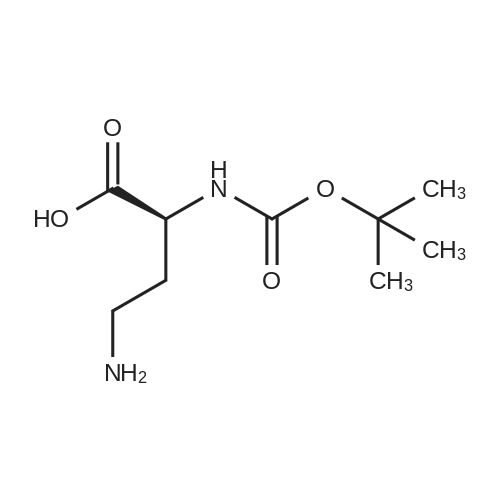
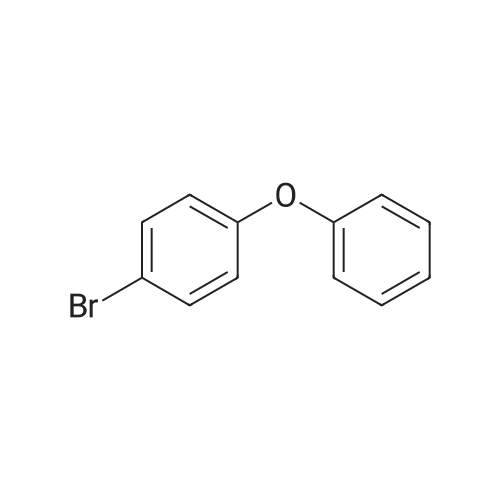
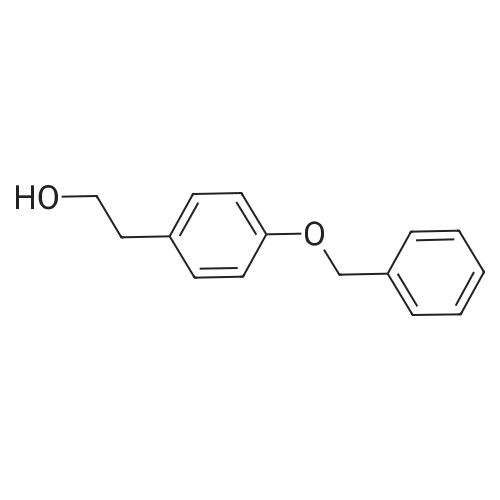
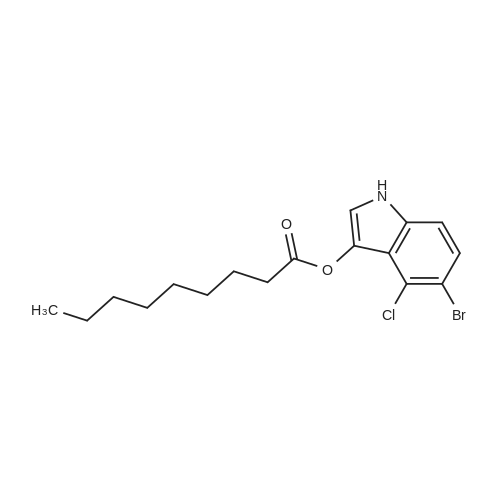
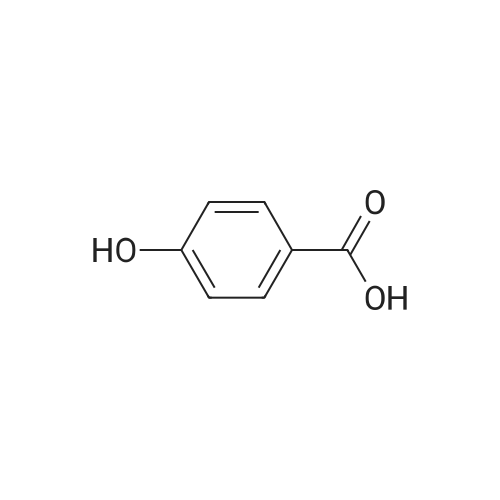
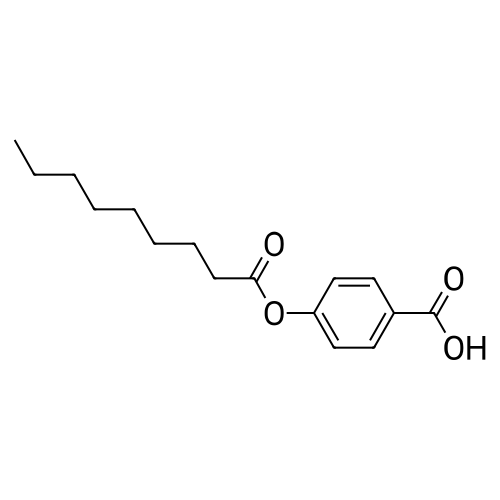
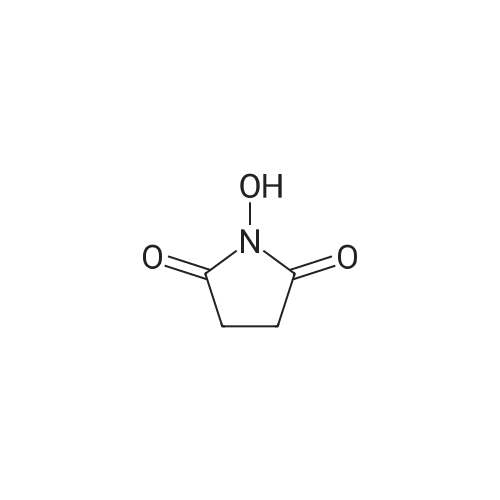
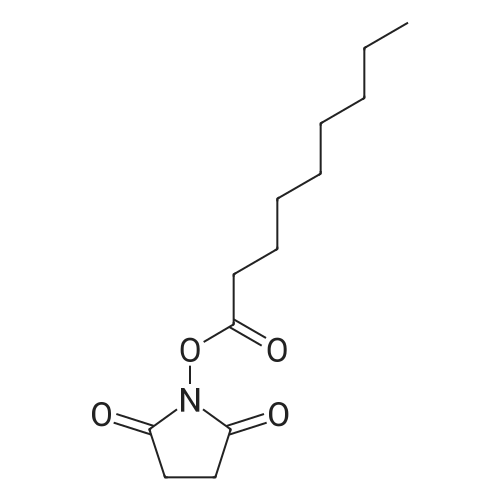
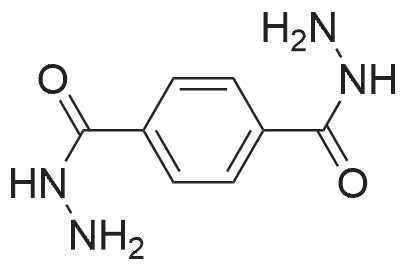
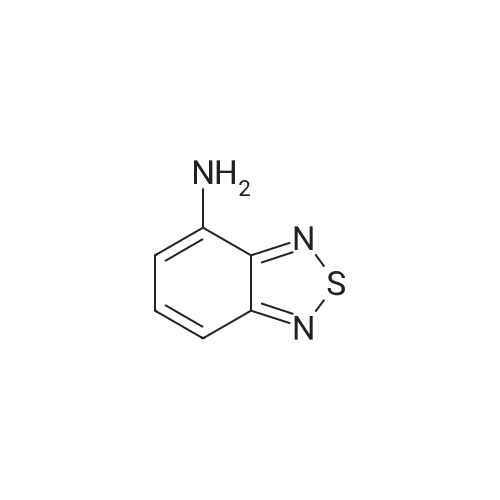
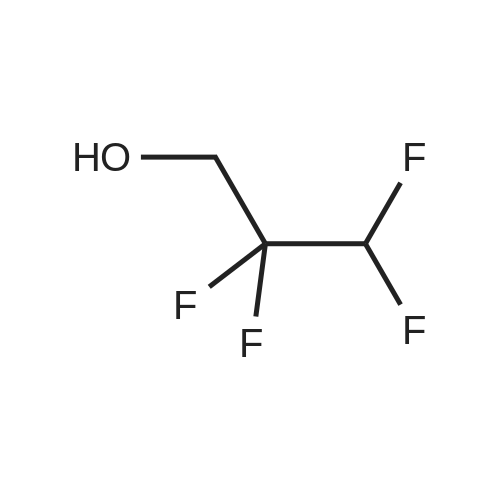
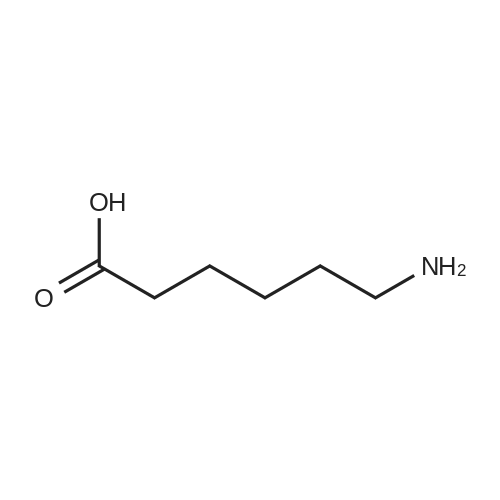
|
With oxalyl dichloride; In dichloromethane; at 0 - 20℃; for 2h; |
A solution of 2-(oxazol-5-yl)pyridine (117 mg, 0.80 mmol) in anhydrous THF (5 mL) cooled to -75 C. under N2 was treated with n-BuLi (2.5 M in hexanes, 1.1 equiv, 0.88 mmol, 0.35 mL), and stirred for 20 min. ZnCl2 (0.5 M in THF, 2.0 equiv, 1.60 mmol, 3.2 mL) was added at -75 C., and stirred for 45 min at 0 C. Cul (1.0 equiv, 0.80 mmol, 152 mg) was added, and the solution was stirred for 10 min at 0 C. A separate flask was charged with nonanoic acid (2 equiv, 1.60 mmol, 253 mg, 0.28 mL) in anhydrous CH2Cl2 (4.2 mL), and to this solution cooled to 0 C. under N2 was added oxalyl chloride (5 equiv, 8.0 mmol, 1.02 g, 0.70 mL). After stirring at rt for 2 h, the solution was concentrated under reduced pressure and dissolved in anhydrous THF (1.5 mL). The solution of nonanoyl chloride was added and the solution was stirred for 1 h at 0 C. The reaction was diluted with EtOAc (10 mL), and washed with 15% aqueous NH4OH (1×10 mL), H2O (1×10 mL), and saturated aqueous NaCl (1×10 mL). The organic layer was dried (Na2SO4), filtered, and concentrated under reduced pressure. Flash chromatography (SiO2, 2.5 cm×17.5 cm, 20% EtOAc-hexanes) afforded 1-(5-(pyridin-2-yl)oxazol-2-yl)nonan-1-one (188) (94 mg, 0.33 mmol, 41% yield) as a light brown powder: mp 56-57 C.; 1H NMR (CDCl3, 250 MHz) d 8.61 (br d, J=4.4 Hz, 1H), 7.84-7.71 (m, 3H), 7.29-7.22 (m, 1H), 3.05 (t, J=7.3 Hz, 2H), 1.79-1.66 (m, 2H), 1.42-1.16 (m, 10H), 0.88-0.77 (m, 3H); 13C NMR (CDCl3, 62.5 MHz) d 188.4, 157.3, 153.1, 150.0, 146.2, 137.0, 126.8, 124.0, 120.3, 39.0, 31.7, 29.2, 29.0, 24.0, 23.9, 22.5, 14.0; IR (KBr) umax 2922, 2856, 1705, 1697, 1600, 1420, 1381 cm-1; MALDI-FTMS (DHB) m/z 287.1744 (C17H22N2O2+H+ requires 287.1754). |
|
With trichloroacetamide; triphenylphosphine; In dichloromethane; for 1h;Reflux; |
General procedure: To a 250-mL round-bottomed flask was added the corresponding carboxylic acid (25 mmol), trichloroacetamide (8.12 g, 50 mmol), triphenylphosphine (13.11 g,50 mmol), and dichloromethane (100 mL) to give a colorless solution. The mixture was stirred and heated at reflux for 1 h. Then, solketal (3.11 mL, 25 mmol) and pyridine (6.04 mL, 75 mmol) were added to the resulting acid chloride solution and the reaction mixture was heated at reflux until completion as indicated by TLC (approximately 4 h). After completion, the solution was extracted with 10% HCl and sat. aq. NaHCO3, dried over Na2SO4 and evaporated. The crude product was purified with a silica gel column eluting with 5% EtOAc/hexane (Scheme 2). |
|
With thionyl chloride;Reflux; |
General procedure: 45 g (50 mL, 0.346 mol) of enanthic acid was slowly added dropwise to 124 g (75 mL,1.042 mol) of thionyl chloride upon reflux. The obtained mixture was heated upon reflux for 30 min, and the excess thionyl chloride was evaporated in vacuum. About 50 g of enanthoyl chloride was obtained as a yellow oil. 25 g (0.102 mol) of 5,7-dimethyl-1,3-bis(hydroxymethyl)adamantane, 70 mL of triethylamine, 50 g (0.337 mol) of enanthoyl chloride, and 200 mL of toluene are placed into a three-neck flask equipped with a mechanical stirrer and a reflux condenser. The obtained mixture is heated upon reflux for 3 h. The formed precipitate of triethylamine hydrochloride is filtered off, and the filtrate is evaporated in vacuum on a rotary evaporator. The residue is purified via vacuum distillation collecting a fraction with bp 200-202C (0.036 Torr). The weight 37 g, yield 74%, |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +

 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping