|
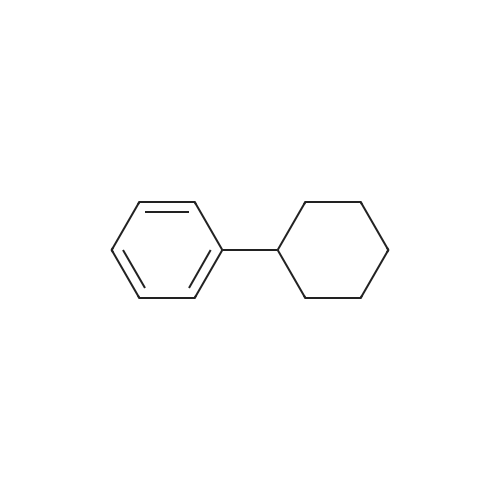
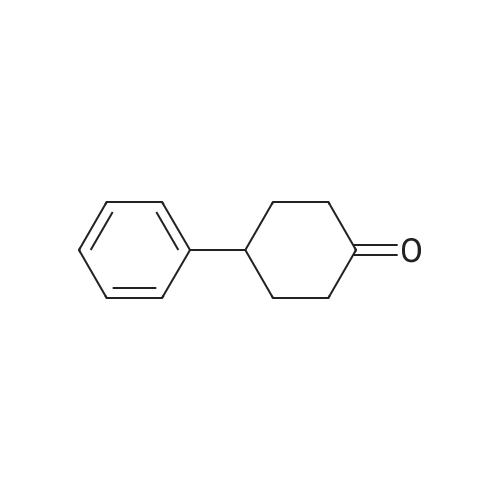
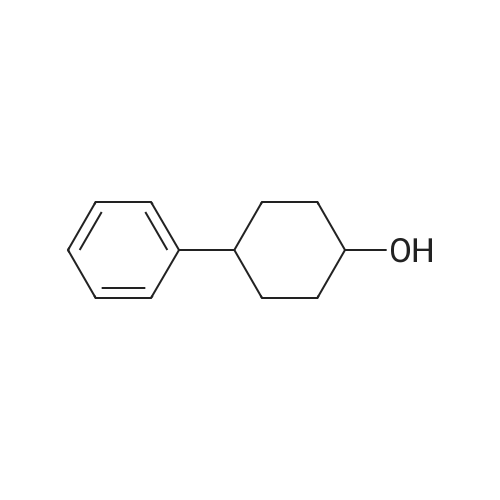
With L-Selectride In tetrahydrofuran at -78℃; for 2h; Inert atmosphere; |
405
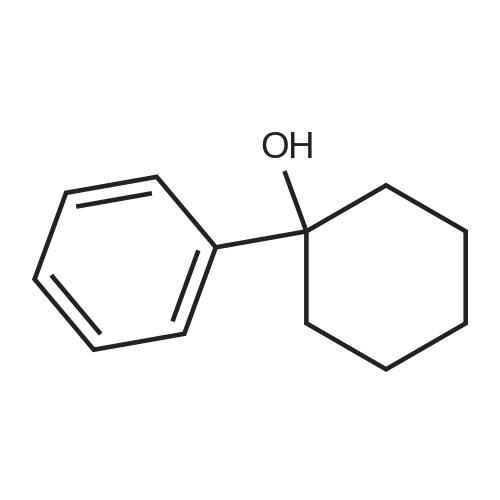
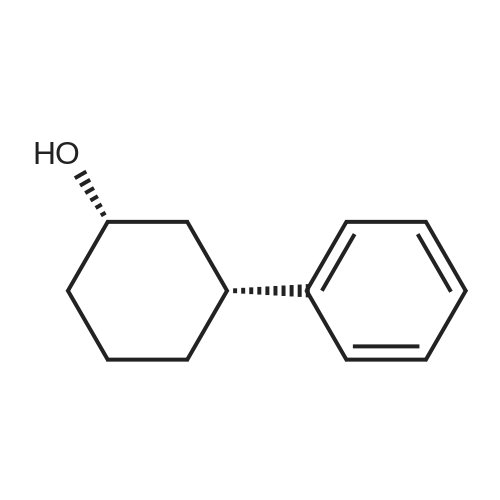
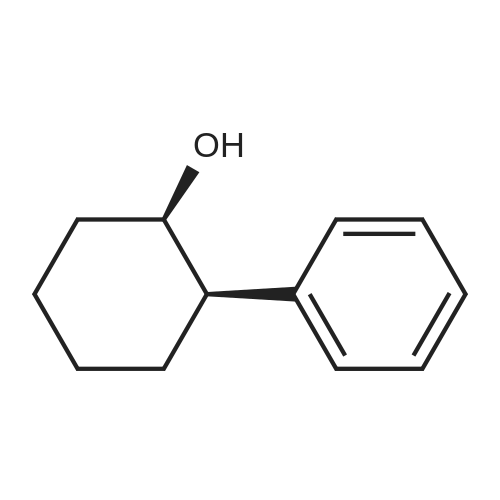
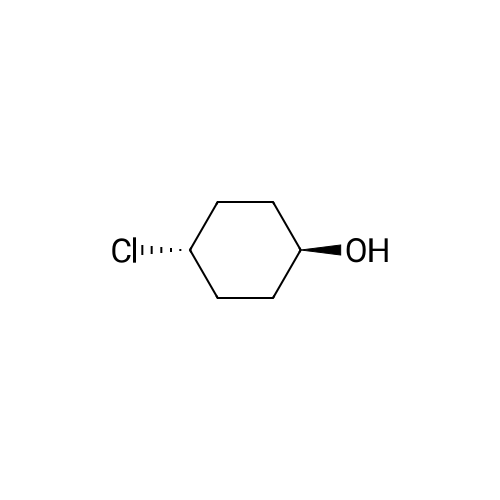
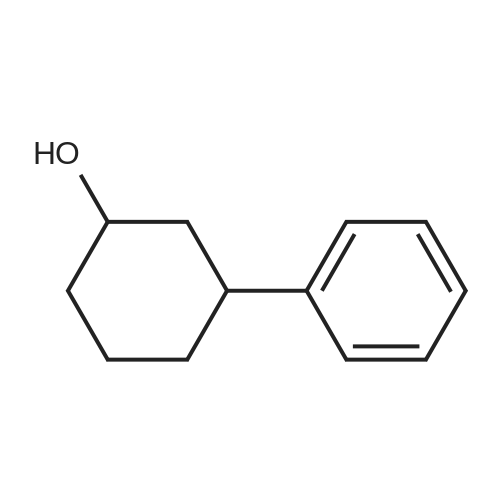
General procedure: To a solution of Compound 393E (820 mg, 2.8 mmol) in anhydrous THF (20 mL) was dropped L-Selectride solution of (1 M in THF, 4.2 mL, 4.2 mmol) at -78 °C under nitrogen and stirred at -78 °C for 2 hours. The reaction mixture was quenched with saturated aqueous NH4CI solution (30 mL), stirred at room temperature for 0.5 hour, and extracted with EtOAc (50 mL x 3). The combined organic layer was dried over anhydrous sodium sulfate, concentrated, and purified with flash column chromatography on silica gel (ethyl acetate in petroleum ether, 10% v/v) to furnish Compounds 393F-1 and 393F-2. Compounds 393F-1: LC-MS (ESI) m/z: 277 [M-OH]+; 1H-NMR (CDCb, 400 MHz): d (ppm) 1.57-1.61 (m, 2H), 1.68-1.73 (m, 2H), 1.82-1.93 (m, 4H), 2.87-2.95 (m, 1H), 4.16-4.17 (m, 1H), 7.14-7.19 (m, 2H), 7.35-7.36 (m, 1H). Compounds 393F-2: LC-MS (ESI) m/z: 277 [M-OH]+; -NMR (CDCb, 400 MHz): d (ppm) 1.34-1.42 (m, 4H), 1.78-1.82 (m, 2H), 2.03-2.05 (m, 2H), 2.78-2.81 (m, 1H), 3.59-3.63 (m, 1H), 7.08-7.17 (m, 2H), 7.18-7.19 (m, 1H). |
|
With L-Selectride In tetrahydrofuran at -78 - 0℃; for 5.33333h; |
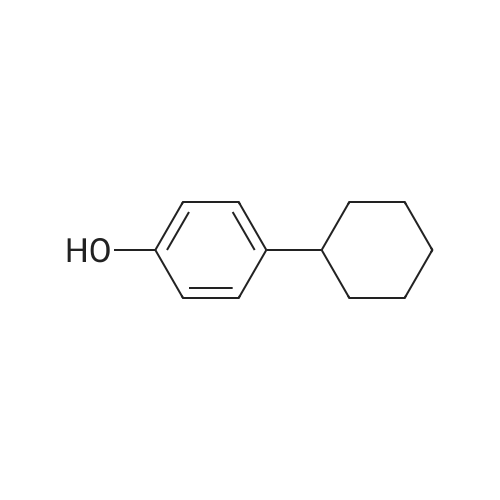
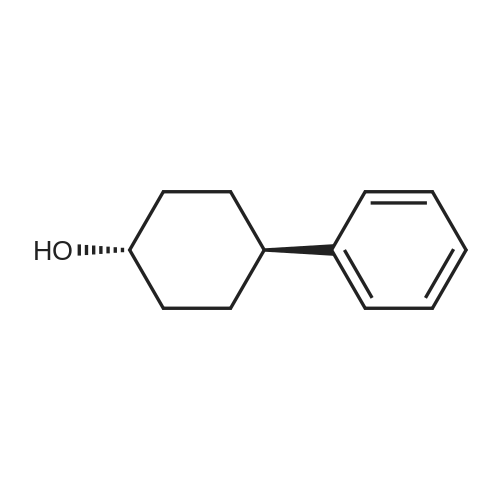
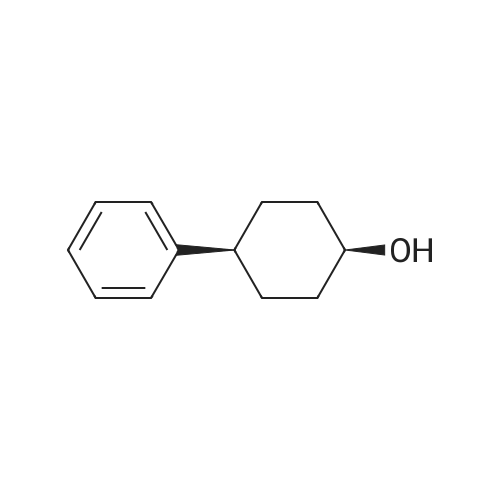
(CIS)-4-phenylcyclohexanol
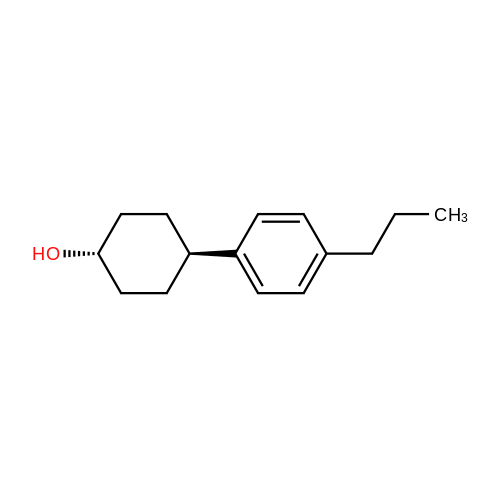
To a mixture of 4-phenylcyclohexanone (10.50 g, 60.3 mmol) in THF (201 mL) at -78° C. was added L-Selectride (102 mL, 102 mmol) in THF over 20 min. The mixture stirred at -78° C. for 3 hours before warming to 0° C. and stirring for an additional 2 hours. The reaction was quenched with a saturated solution of NH4Cl (200 mL), extracted with EtOAc (250 mL×3), dried over Na2SO4, and concentrated. The residue was purified by column chromatography on silica (2% to 60% EtOAc/hexanes) to afford the title compound. MS: 199.9 (M+23). |
|
With L-Selectride In tetrahydrofuran at -78℃; for 2h; Inert atmosphere; |
405
General procedure: To a solution of Compound 393E (820 mg, 2.8 mmol) in anhydrous THF (20 mL) was dropped L-Selectride solution of (1 M in THF, 4.2 mL, 4.2 mmol) at -78 oC under nitrogen and stirred at -78 oC for 2 hours. The reaction mixture was quenched with saturated aqueous NH4Cl solution (30 mL), stirred at room temperature for 0.5 hour, and extracted with EtOAc (50 mL x 3). The combined organic layer was dried over anhydrous sodium sulfate, concentrated, and purified with flash column chromatography on silica gel (ethyl acetate in petroleum ether, 10% v/v) to furnish Compounds 393F-1 and 393F-2. Compounds 393F-1: LC-MS (ESI) m/z: 277 [M-OH]+; 1H-NMR (CDCl3, 400 MHz): d (ppm) 1.57-1.61 (m, 2H), 1.68-1.73 (m, 2H), 1.82-1.93 (m, 4H), 2.87-2.95 (m, 1H), 4.16-4.17 (m, 1H), 7.14-7.19 (m, 2H), 7.35-7.36 (m, 1H). Compounds 393F-2: LC-MS (ESI) m/z: 277 [M-OH]+; 1H-NMR (CDCl3, 400 MHz): d (ppm) 1.34-1.42 (m, 4H), 1.78-1.82 (m, 2H), 2.03-2.05 (m, 2H), 2.78-2.81 (m, 1H), 3.59-3.63 (m, 1H), 7.08-7.17 (m, 2H), 7.18-7.19 (m, 1H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping