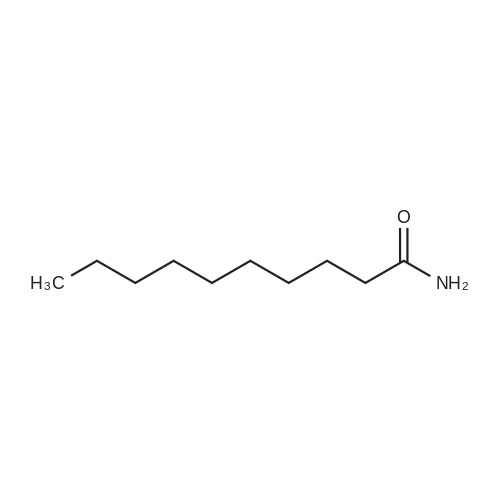
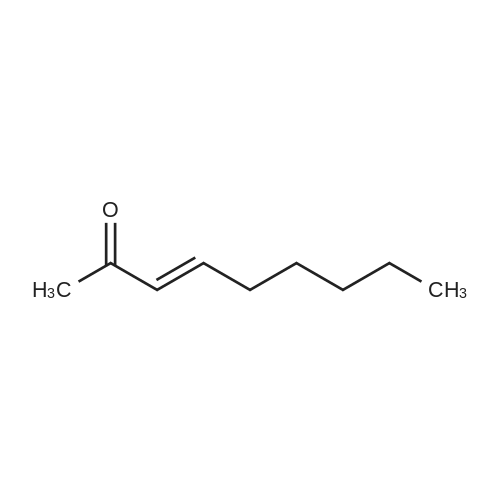
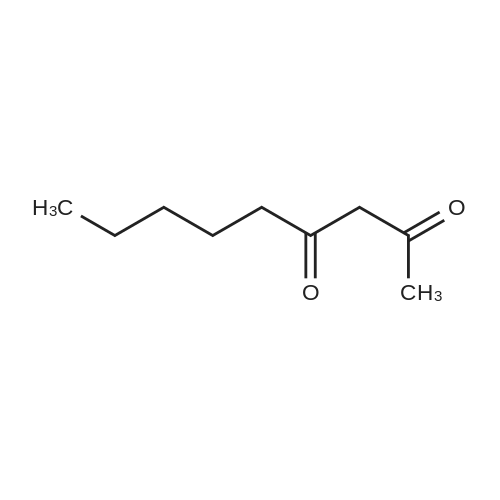
|
With dihydrogen peroxide;lithium hydroxide; In tert-Amyl alcohol; water; at 110℃; for 6h;Heating / reflux; |
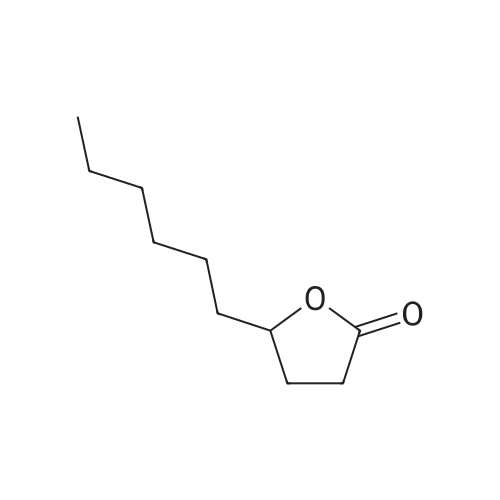
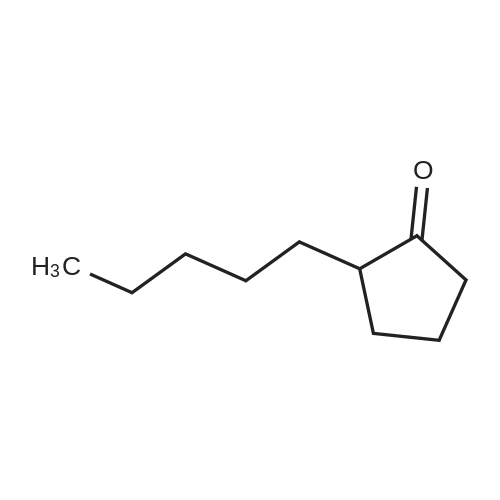
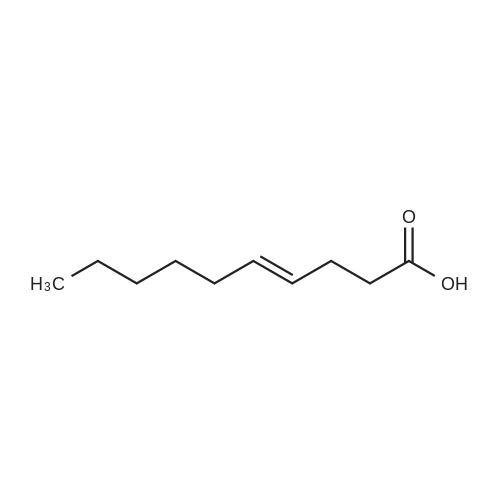
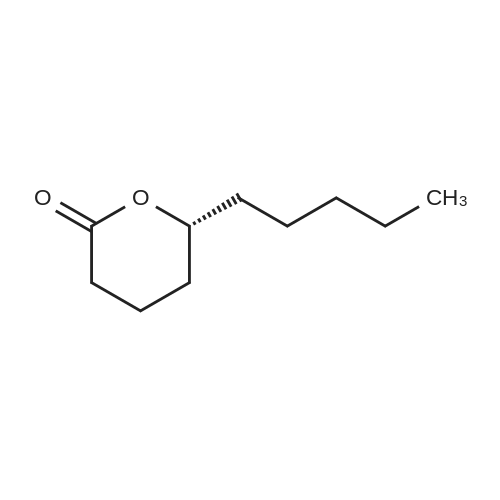
This example illustrates the influence of the catalyst USED IN THE BAEYER-VILLIGER OXIDATION of 2-pentyl cyclopentanone to lactones by 202. The reaction was carried out in the presence of 1 mol% catalyst in ethyl propionate as solvent at 110C in the same conditions as those reported in Example 1, Procedure C). The results are reported in Table 1.When the reaction was carried out in the presence of 1 mol% catalyst in tert-amyl alcohol as solvent at reflux in the same conditions as those reported in Example 1, Procedure C), similar results, reported in Table LA, were obtained. |
|
With dihydrogen peroxide;sodium hydrogencarbonate; In water; Ethyl propionate; at 110℃; for 6h;Heating / reflux; |
This example illustrates the influence of the catalyst USED IN THE BAEYER-VILLIGER OXIDATION of 2-pentyl cyclopentanone to lactones by 202. The reaction was carried out in the presence of 1 mol% catalyst in ethyl propionate as solvent at 110C in the same conditions as those reported in Example 1, Procedure C). The results are reported in Table 1. |
|
With dihydrogen peroxide;sodium hydrogencarbonate; In tert-Amyl alcohol; water; at 110℃; for 6h;Heating / reflux; |
This example illustrates the influence of the catalyst USED IN THE BAEYER-VILLIGER OXIDATION of 2-pentyl cyclopentanone to lactones by 202. The reaction was carried out in the presence of 1 mol% catalyst in ethyl propionate as solvent at 110C in the same conditions as those reported in Example 1, Procedure C). The results are reported in Table 1.When the reaction was carried out in the presence of 1 mol% catalyst in tert-amyl alcohol as solvent at reflux in the same conditions as those reported in Example 1, Procedure C), similar results, reported in Table LA, were obtained. |
|
With dihydrogen peroxide;magnesium 2-ethylhexanoate; In water; Ethyl propionate; at 110℃; for 6h;Heating / reflux; |
This example illustrates the influence of the catalyst USED IN THE BAEYER-VILLIGER OXIDATION of 2-pentyl cyclopentanone to lactones by 202. The reaction was carried out in the presence of 1 mol% catalyst in ethyl propionate as solvent at 110C in the same conditions as those reported in Example 1, Procedure C). The results are reported in Table 1. |
|
With dihydrogen peroxide;sodium hydroxide; In tert-Amyl alcohol; water; at 110℃; for 6h;Heating / reflux; |
This example illustrates the influence of the catalyst USED IN THE BAEYER-VILLIGER OXIDATION of 2-pentyl cyclopentanone to lactones by 202. The reaction was carried out in the presence of 1 mol% catalyst in ethyl propionate as solvent at 110C in the same conditions as those reported in Example 1, Procedure C). The results are reported in Table 1.When the reaction was carried out in the presence of 1 mol% catalyst in tert-amyl alcohol as solvent at reflux in the same conditions as those reported in Example 1, Procedure C), similar results, reported in Table LA, were obtained. |
|
With dihydrogen peroxide;lithium bromide; In water; Ethyl propionate; at 110℃; for 6h;Heating / reflux; |
This example illustrates the influence of the catalyst USED IN THE BAEYER-VILLIGER OXIDATION of 2-pentyl cyclopentanone to lactones by 202. The reaction was carried out in the presence of 1 mol% catalyst in ethyl propionate as solvent at 110C in the same conditions as those reported in Example 1, Procedure C). The results are reported in Table 1. |
|
With dihydrogen peroxide;potassium acetate; In water; Ethyl propionate; at 110℃; for 6h;Heating / reflux; |
This example illustrates the influence of the catalyst USED IN THE BAEYER-VILLIGER OXIDATION of 2-pentyl cyclopentanone to lactones by 202. The reaction was carried out in the presence of 1 mol% catalyst in ethyl propionate as solvent at 110C in the same conditions as those reported in Example 1, Procedure C). The results are reported in Table 1. |
|
With dihydrogen peroxide;sodium 2,2,2-trifluoroacetate; In water; Ethyl propionate; at 110℃; for 6h;Heating / reflux; |
This example illustrates the influence of the catalyst USED IN THE BAEYER-VILLIGER OXIDATION of 2-pentyl cyclopentanone to lactones by 202. The reaction was carried out in the presence of 1 mol% catalyst in ethyl propionate as solvent at 110C in the same conditions as those reported in Example 1, Procedure C). The results are reported in Table 1. |
|
With dihydrogen peroxide;sodium 2-ethylhexanoic acid; In water; Ethyl propionate; at 110℃; for 6h;Heating / reflux; |
This example illustrates the influence of the catalyst USED IN THE BAEYER-VILLIGER OXIDATION of 2-pentyl cyclopentanone to lactones by 202. The reaction was carried out in the presence of 1 mol% catalyst in ethyl propionate as solvent at 110C in the same conditions as those reported in Example 1, Procedure C). The results are reported in Table 1. |
|
With dihydrogen peroxide;lithium acetate; In water; chlorobenzene; at 110℃; for 6h;Heating / reflux;Reactivity; |
This example illustrates the influence of the solvent used in the Baeyer-Villiger oxidation of 2-pentyl cyclopentanone to lactones by 70 % w/w aqueous H202 solution at 110C and in the presence of 1 mol% lithium acetate. The reaction was carried out under the same conditions as those described in Example 1, Procedure C). The results are reported in Table 2. |
|
With dihydrogen peroxide;lithium acetate; In 1,4-dioxane; water; at 110℃; for 6h;Heating / reflux;Reactivity; |
This example illustrates the influence of the solvent used in the Baeyer-Villiger oxidation of 2-pentyl cyclopentanone to lactones by 70 % w/w aqueous H202 solution at 110C and in the presence of 1 mol% lithium acetate. The reaction was carried out under the same conditions as those described in Example 1, Procedure C). The results are reported in Table 2. |
|
With dihydrogen peroxide;lithium acetate; In Isopropyl acetate; water; at 110℃; for 6h;Heating / reflux;Reactivity; |
This example illustrates the influence of the solvent used in the Baeyer-Villiger oxidation of 2-pentyl cyclopentanone to lactones by 70 % w/w aqueous H202 solution at 110C and in the presence of 1 mol% lithium acetate. The reaction was carried out under the same conditions as those described in Example 1, Procedure C). The results are reported in Table 2. |
|
With dihydrogen peroxide;lithium acetate; In water; Ethyl propionate; at 110℃; for 6h;Heating / reflux; |
This example illustrates the influence of the catalyst USED IN THE BAEYER-VILLIGER OXIDATION of 2-pentyl cyclopentanone to lactones by 202. The reaction was carried out in the presence of 1 mol% catalyst in ethyl propionate as solvent at 110C in the same conditions as those reported in Example 1, Procedure C). The results are reported in Table 1.Example 3 BAEYER-VILLIGER Oxidation of pentyl cyclopentanone using various solvents This example illustrates the influence of the solvent used in the Baeyer-Villiger oxidation of 2-pentyl cyclopentanone to lactones by 70 % w/w aqueous H202 solution at 110C and in the presence of 1 mol% lithium acetate. The reaction was carried out under the same conditions as those described in Example 1, Procedure C). The results are reported in Table 2. |
|
With dihydrogen peroxide;lithium acetate; In tert-Amyl methyl ether; water; at 110℃; for 6h;Heating / reflux;Reactivity; |
This example illustrates the influence of the solvent used in the Baeyer-Villiger oxidation of 2-pentyl cyclopentanone to lactones by 70 % w/w aqueous H202 solution at 110C and in the presence of 1 mol% lithium acetate. The reaction was carried out under the same conditions as those described in Example 1, Procedure C). The results are reported in Table 2. |
|
With dihydrogen peroxide;calcium carbonate; In water; Ethyl propionate; at 110℃; for 6h;Heating / reflux; |
This example illustrates the influence of the catalyst USED IN THE BAEYER-VILLIGER OXIDATION of 2-pentyl cyclopentanone to lactones by 202. The reaction was carried out in the presence of 1 mol% catalyst in ethyl propionate as solvent at 110C in the same conditions as those reported in Example 1, Procedure C). The results are reported in Table 1. |
|
With dihydrogen peroxide;sodium peroxide; In water; Ethyl propionate; at 110℃; for 6h;Heating / reflux; |
This example illustrates the influence of the catalyst USED IN THE BAEYER-VILLIGER OXIDATION of 2-pentyl cyclopentanone to lactones by 202. The reaction was carried out in the presence of 1 mol% catalyst in ethyl propionate as solvent at 110C in the same conditions as those reported in Example 1, Procedure C). The results are reported in Table 1. |
|
With dihydrogen peroxide;Na(polyacrylate); In water; Ethyl propionate; at 110℃; for 6h;Heating / reflux; |
This example illustrates the influence of the catalyst USED IN THE BAEYER-VILLIGER OXIDATION of 2-pentyl cyclopentanone to lactones by 202. The reaction was carried out in the presence of 1 mol% catalyst in ethyl propionate as solvent at 110C in the same conditions as those reported in Example 1, Procedure C). The results are reported in Table 1. |
|
With dihydrogen peroxide;lithium pentane-2,4-dionate; In water; Ethyl propionate; at 110℃; for 6h;Heating / reflux; |
This example illustrates the influence of the catalyst USED IN THE BAEYER-VILLIGER OXIDATION of 2-pentyl cyclopentanone to lactones by 202. The reaction was carried out in the presence of 1 mol% catalyst in ethyl propionate as solvent at 110C in the same conditions as those reported in Example 1, Procedure C). The results are reported in Table 1. |
|
With dihydrogen peroxide;lithium carbonate; In water; Ethyl propionate; at 110℃; for 6h;Heating / reflux; |
This example illustrates the influence of the catalyst USED IN THE BAEYER-VILLIGER OXIDATION of 2-pentyl cyclopentanone to lactones by 202. The reaction was carried out in the presence of 1 mol% catalyst in ethyl propionate as solvent at 110C in the same conditions as those reported in Example 1, Procedure C). The results are reported in Table 1. |
|
With dihydrogen peroxide;barium carbonate; In water; Ethyl propionate; at 110℃; for 6h;Heating / reflux; |
This example illustrates the influence of the catalyst USED IN THE BAEYER-VILLIGER OXIDATION of 2-pentyl cyclopentanone to lactones by 202. The reaction was carried out in the presence of 1 mol% catalyst in ethyl propionate as solvent at 110C in the same conditions as those reported in Example 1, Procedure C). The results are reported in Table 1. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping