Alternatived Products of [ 68892-13-7 ]
Product Details of [ 68892-13-7 ]
| CAS No. : | 68892-13-7 |
MDL No. : | MFCD09025938 |
| Formula : |
C16H22O2
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | - |
| M.W : |
246.34
|
Pubchem ID : | - |
| Synonyms : |
|
Application In Synthesis of [ 68892-13-7 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 68892-13-7 ]
- 1
-
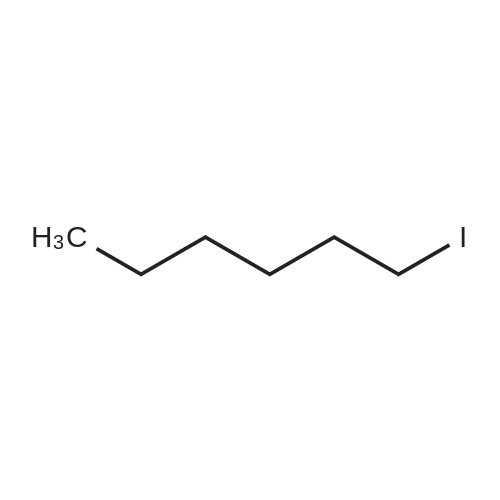 [ 638-45-9 ]
[ 638-45-9 ]

-
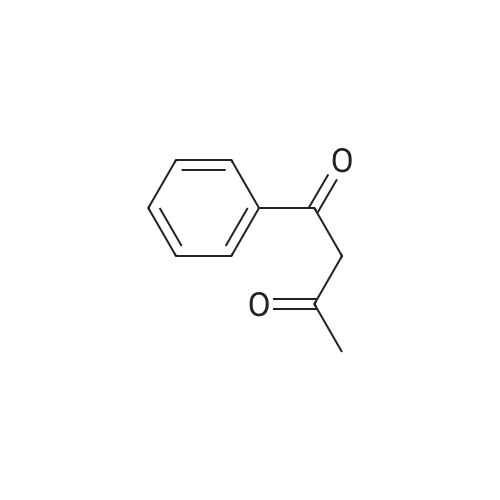 [ 93-91-4 ]
[ 93-91-4 ]

-
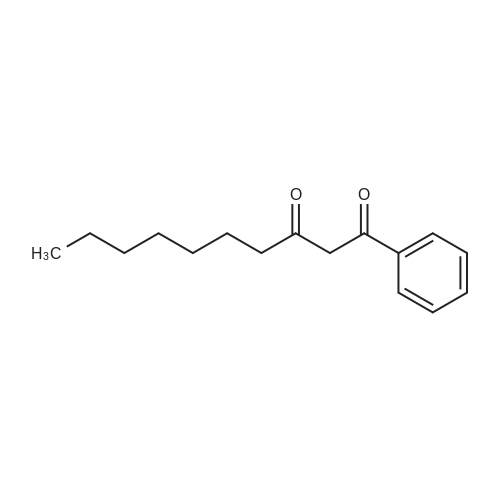 [ 68892-13-7 ]
[ 68892-13-7 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With diisopropylamine In tetrahydrofuran |
Intermediate 19: 1(phenyl)-1,3decanedione
Intermediate 19: 1(phenyl)-1,3decanedione To a solution of 1 g (6.1 mmol) of benzoylacetone in 6 mL of THF at -78° C. was added 8.2 mL (12.3 mmol) of a 1.5 M LDA solution in THF. Warmed to 0° C. over 2 h, then 2.6 mL (12.3 mmol) of 1-iodohexane was added. The mixture was stirred at rt for 3 h before quenching with saturated aqueous NH4Cl solution and dilution with Et2O. The organics were dried (Na2SO4), filtered, concentrated, and purified by silica gel chromatography (15/1 hexanes/Et2O) to yield Intermediate 19: TLC Rf=0.52 (1/10 Et2O/Hexanes); 1H NMR (400 MHz, CDCl3, enol form) δ7.88 (m, 2H), 7.54-7.43 (m, 3H), 6.17 (s, 1H), 2.42 (t, 2H, J=7.6), 1.66 (m, 2H), 1.4-1.25 (m, 8H), 0.85 (m, 3H); low resolution MS(ES+) m/e 269.0 (M+Na+). |
- 2
-
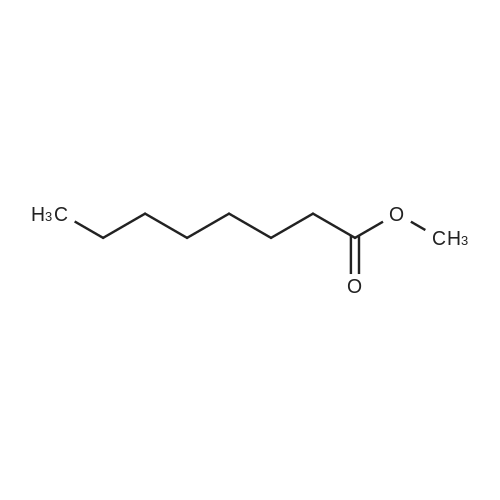 [ 111-11-5 ]
[ 111-11-5 ]

-
 [ 98-86-2 ]
[ 98-86-2 ]

-
 [ 68892-13-7 ]
[ 68892-13-7 ]
| Yield | Reaction Conditions | Operation in experiment |
| 98.3% |
With sodium methylate In 5,5-dimethyl-1,3-cyclohexadiene for 1.25h; Inert atmosphere; Reflux; Microwave irradiation; |
7 EXAMPLE 7Synthesis of OctanoylBenzoylMethane (OBM) usingthe Process According to the Invention
10130] The experimental apparatus consists of a classic glass, double jacketed chemical engineering reactor with a volume of 1 litre with an effective mixing system. This is topped with a separating column fitted with a variable-reflux condenser. It also has a recirculating loop fitted with a geartype pump and a 600 W microwave generatot 10131] Add 550 mE xylenes, 94.78 g fused methyl octanoate and 34.05 g powdered sodium methoxide. Once the reagents have been added, render the reactor inert with a continuous flow of nitrogen gas. Recirculate the mixture through the external circuit at a rate of 15 kg/h. Bring to boiling and total reflux, and then switch the microwave sourceon.10132] Add 68.41 g acetophenone over one hout Once it has all been added, let the reaction continue for another 15 minutes. Throughout all this time, draw the methanol off the reaction mixture. After the 15 minutes of finishing time, switch off the microwave source and the heatet AcidiFy the mixture and then wash it.10133] Analysis of the organic phase by gas phase chromatography shows that almost all the acetophenone is consumed and that the OBM titre is 98.3%. OBM productivity during the reaction phase is 110.3 kg/hIm3. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping








