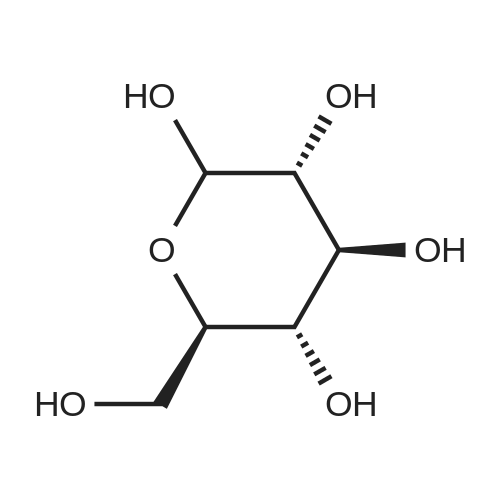
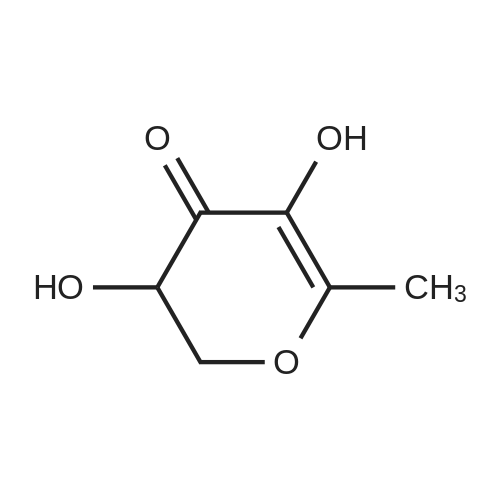
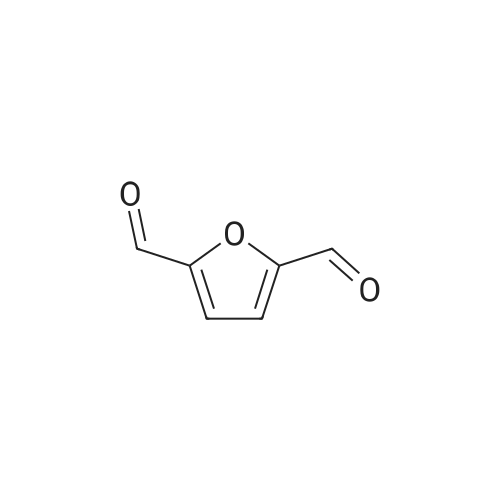
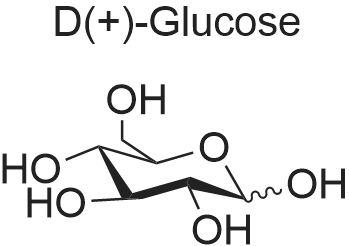
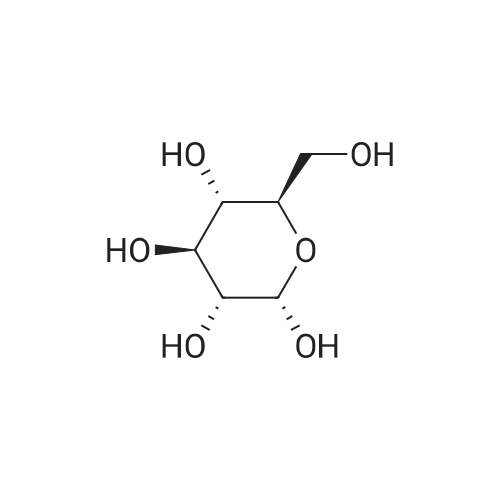
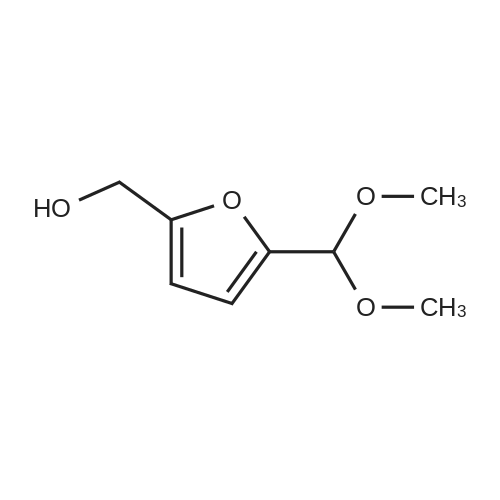
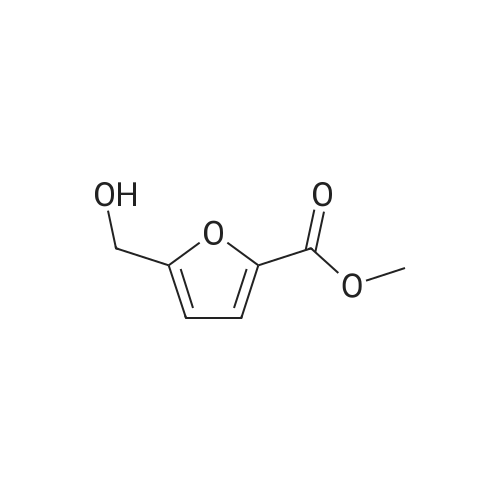
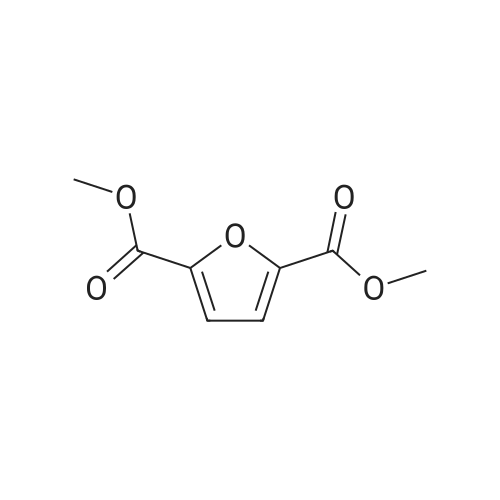
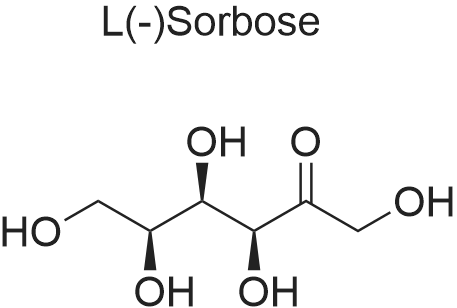
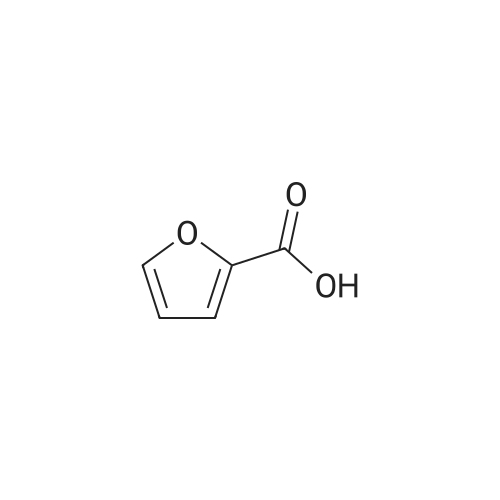
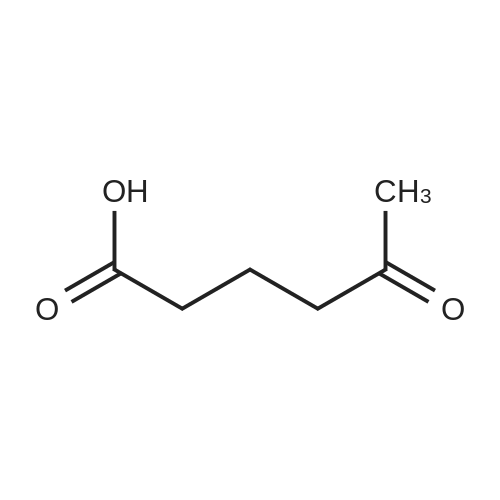
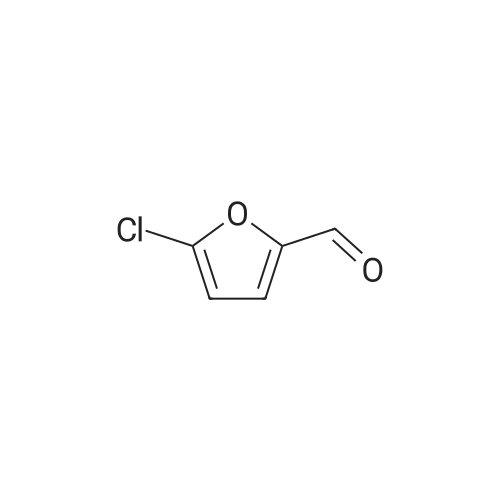
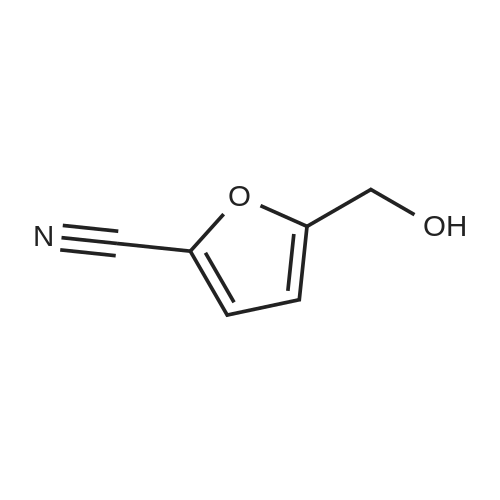
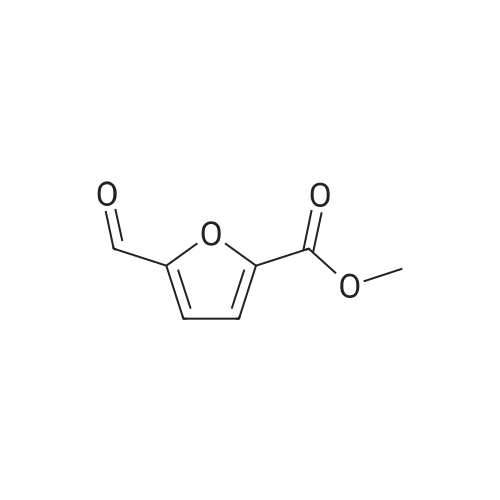
| 98% |
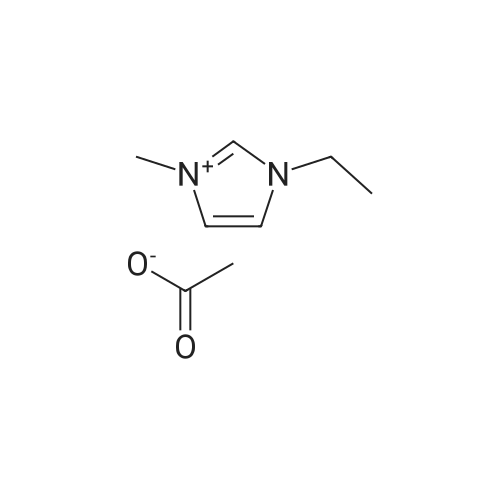
With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; oxygen; In acetic acid; at 50℃; under 760.051 Torr; for 5.0h; |
The experiments were carried out in test tubes equipped with a balloon containing pure O2, using O2 as the oxidant. In a general procedure, HMF (125 mg, 1 mmol), ECS-IL-Al(NO3)3 (90 mg), TEMPO (8.5 mg, 0.05 mmol) and glacial acetic acid (2 mL) were added to a 10 mL test tube. The reaction mixture was stirred magnetically at 50 C for 5 h. After completion of the reaction, the mixture was cooled down to room temperature and filtered, and the remaining solid was washed with acetonitrile and acetone to separate the catalyst. Then, the recovered catalyst was dried and used in several runs in the same manner as reported for the first run. Samples were collected and analyzed via HPLC. |
| 96.6% |
With manganese(IV) oxide; In toluene;Dean-Stark; Inert atmosphere; Heating; |
To a single necked (24/40) round bottomed flask was added, toluene (400 mL), 5-hydroxymethylfurfural (11.46 g, 0.0909 mol), and a PTFE coated magnetic spinning egg. The mixture was stirred vigorously pure-crystalline HMF did not fully dissolve. To that stirring mixture was added 88% active electrolytically precipitated manganese dioxide (Alfa Aesar, 11.51 g, 0.2498 mol). The black slurry was stirred vigorously, and a Dean-Stark trap was installed above the flask (topped with a Dimroth condenser plumbed with a stream of cold water). The headspace of the flask was purged with argon and the mixture was brought up to a fast boil. The flask was wrapped in aluminum foil and distillate began to collect. As the reaction proceeded, water separated to the bottom of the trap while dry toluene was allowed to return. This azeotropic distillation proceeded for six hours. The mixture was suction filtered through qualitative paper in a ceramic Buchner funnel. The residue was packed into a Soxhlet extractor and the filtrate was installed below. The Soxhlet extractor was charged from the top with enough acetone to flush the extractor five times. The mixture was brought up to boiling temperature with a heating mantle and a variable controller. The residue was continuously extracted with acetone in that manner overnight. (0148) The heat was killed, the acetone/toluene solution was suction filtered through a bed of Celite packed into a medium porosity fitted Buchner funnel. The filtrated was a light yellow color and was concentrated by rotary evaporation under a vacuum induced by a water aspirator. The light yellow crystalline solid was scraped into a free flowing flakey solid and dried on the high vacuum line to constant mass which afforded 2,5-diformyl furan (10.89 g, 0.0878 mol, 96.6% yield). 1H NMR (CDCl3, 400 MHz) delta: 7.35 (s, 2H), 9.87 (s, 2H) (0150) 13C NMR (CDCl3 100 MHz) delta: 119.3, 154.2, 179.2 |
| 95.3% |
With oxygen; In dimethyl sulfoxide; at 120℃; under 7500.75 Torr; for 6.0h; |
In atypical experiment, the high-pressure hastelloy reactor(Microreactor, Yanzheng Instrument Ltd. Shanghai) was charged with HMF (126 mg, 1 mmol), HPMoVsurf(n)/CeO2 (80 mg), and 5 mLDMSO. The reaction was conducted at 120 C and initiated by vigorous stirring with a magnetic stirrer under 1.0 MPa of oxygen pressure for 6 h. After the reaction was finished, the reactor was cooling down toroom temperature and the oxygen pressure was released. The catalyst was separated by centrifugation. The analysis of the recovered solutions was monitored by using high-performance liquid chromatography(HPLC). Samples were separated by a reversed-phase C18 column and detected by UV detector at the wavelength of 280 nm. The mobile phase was constituted of acetonitrile and 0.1 wt % acetic acid aqueous solution(10: 90, v/v) at 0.9 mL min-1 [18]. |
| 95% |
With 3-(tert-butoxycarbonyl amino)-9-azabicyclo[3.3.1]nonane N-oxyl; oxygen; sodium nitrite; In acetic acid; at 25℃; for 1.0h; |
General procedure: A 25-mL tube equipped with a magnetic stirrer bar was added p-methylbenzyl alcohol (0.122 g, 1 mmol), sodium nitrite (5.5 mg, 8 mol%) and 3-(tert-butoxycarbonyl amino)-9-azabicyclo[3.3.1]nonane N-oxyl (3-BocNH-ABNO) (7.7 mg, 3 mol%). After the air in the tube was replaced with O2, 1 mL of acetic acid was added with syringe. Then the mixture was stirred under dioxygen atmosphere (balloon) at room temperature until the reaction was completed. After the reaction was finished, to the reaction mixture was added 8 mL of diethyl ether. Then the mixture was transferred into a separation funnel, and washed with saturated sodium bicarbonate solution (10 mL×3). The aqueous phase was extracted with 8 mL of ether. The combined organic phases was concentrated on a rotary evaporator and the residue was purified by column chromatography on silica gel using petroleumether/diethyl ether as eluent to afford p-methyl benzaldehyde as a colorless liquid; yield: 0.108 g (90%). |
| 94.7% |
With 4-acetylamino-2,2,6,6-tetramethyl-1-piperidinoxy; oxygen; nitric acid; In toluene; at 85℃; under 7500.75 Torr; for 2.5h;Autoclave; |
Add 5-hydroxymethylfurfural (5-HMF, 2.5 g) to toluene (47.5 g) to a concentration of 5% by mass in an autoclave with an internal volume of 100 mL,Furthermore 4-acetamide-2,2,6,6-Tetramethylpiperidine 1-oxyl(4AA-TEMPO, 0.106 g, 2.5 mol% relative to 5-HMF) and 1N nitric acid (2.07 g) were added and sealed. Using a back pressure valve at the outlet of the autoclave while passing a mixed gas of 5% by volume oxygen gas and 95% by volume nitrogen gas as the oxygen-containing gas at a rate of 100 mL / min, the pressure in the autoclave is 1.0 MPa Adjusted to The reaction solution was reacted at 85 C. for 2.5 hours while being stirred at 500 rpm. The outlet oxygen concentration was measured with an oximeter manufactured by Itinenjiko Co., Ltd., and the point at which the consumption of oxygen was not confirmed was taken as the end point of the reaction.After the reaction, the reaction solution is analyzed using a gas chromatograph (column: DB-1701, detector: FID) manufactured by Agilent, and the raw material conversion ratio, the yield of diformylfuran (DFF), the selectivity, the DFF production rate The catalyst rotation number (TON) was calculated. Since the ratio of the amount of nitric acid to toluene, which is an organic solvent, is small, the reaction solution was diluted and analyzed without separating the aqueous layer and the organic layer. In addition, the DFF formation rate represents the number of moles of DFF produced per hour by oxidation of 5-HMF by 1 mole of 4AA-TEMPO, and the catalyst rotation number is 5-HMF of 1 mole of 4AA-TEMPO. Represents the number of moles of DFF produced by oxidizing. The results are shown in Table 1.The reaction solution was also cooled in an ice bath for about 1 hour. After cooling, the reaction solution was treated with ADVANTEC No. The crystals were collected by vacuum filtration using 5 C filter paper and a Buchner funnel. The obtained crystals were analyzed by gas chromatography, which confirmed that the DFF had a purity of over 98%. In addition, the recovery rate of DFF was 72.4%. |
| 93% |
With 2,4,6-trimethyl-pyridine; 4-acetylamino-2,2,6,6-tetramethyl-1-piperidinoxy; iodine; sodium hydrogencarbonate; In dichloromethane; water; at 20 - 22℃; for 1.0h;Catalytic behavior; |
A solution of 5-hydroxylmethylfurfural 1c (10.08 g, 80 mmol), nitroxide 4a (0.85 g, 4.0 mmol) and collidine 6d (0.97 g, 8 mmol) in CH2Cl2 (100 mL) was added to a vigorously stirred solution of NaHCO3 (20.16 g, 240 mmol) in water (100 mL) at 20 C. Then I2 (40.6 g, 160 mmol) powder was added portionwise within 10 min to the formed reaction mixture at vigorous stirring and temperature 20-22 C. The reaction mixture was stirred at 20-22 C for 1 h, then powdered crystalline sodium thiosulfate was added portionwise to discoloration. Organic and aqueous phases were separated and the aqueous phase was then extracted with CH2Cl2 (3×20 mL). Organic phase and the extracts were combined, dried with anhydrous Na2SO4 and evaporated to dryness to give 9.22 g of crude 2c of 97% purity according to HPLC analysis (93% yield). This product was recrystallized from water to give 8.37 g (88% yield) of pure compound 2c, mp 109-110 C (lit.1 109-110 C). 1H NMR (CDCl3) delta 7.33 (s, 2H, 2CH), 9.83 (s, 2H, 2CHO). 13C NMR (CDCl3) delta 119.4, 154.3, 179.3. |
| 93% |
With phosphoric acid; sodium nitrite; at 25℃; for 1.0h; |
Sodium nitrite was added with vigorous stirring (magnetic stirrer) to a solutionof 0.310 g (2.46 mmol) of 5-(hydroxymethyl)-furfural in 4 mL of phosphoric acid (85 wt %). Themixture was stirred for 1 h at 25C in a flask equippedwith a reflux condenser, 10 mL of distilled water wasadded, and the mixture was extracted with chloroform(5 × 5 mL). The combined extracts were washed withwater until neutral washings, dried over anhydroussodium sulfate, filtered, and evaporated to dryness.The residue was recrystallized from hexane containing2 vol % of chloroform. Colorless crystals, mp 108-110C [9]. 1H NMR spectrum, delta, ppm: 7.33 s (2H,=CH), 9.83 s (2H, CHO). 13C-{1H} NMR spectrum,deltaC, ppm: 119.4 (=CH), 154.2 (=C), 179.2 (CHO). |
| 90% |
With Iron(III) nitrate nonahydrate; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; sodium chloride; In 1,2-dichloro-ethane; at 20℃; for 4.0h; |
A, 5-HMF (63 mg, 0.5 mmol) was added to 2 mL of dichloroethane and then TEMPO (7.8 mg, 0.025 mmol, 5 mol%), Fe (NO3)3· 9H2(10.1 mg, 0.025 mmol, 5 mol%) and NaCl (1.5 mg, 0.025 mol, 5 mol%) were added and stirred at room temperature for 4 h to give a reaction mixture containing 2,5-furanaldehyde. The results of the GC detection are shown in Fig. 1. It can be seen from Fig. 1 that the product 2,5-furanaldehyde (internal standard: 5-methylfurfural) was successfully obtained. |
| 88% |
With Iron(III) nitrate nonahydrate; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; sodium chloride; In 1,2-dichloro-ethane; at 20℃; for 4.0h;Catalytic behavior; |
5-HMF (63 mg, 0.5 mmol), Fe(NO3)3·9H2O (10.1 mg, 0.025 mmol, 5 mol%), TEMPO (7.8 mg, 0.025 mmol, 5 mol%), NaCl (1.5 mg, 0.025 mol, 5 mol%) were charged into a tube, and then 2 mL of DCE was added. The reaction mixture was stirred at room temperature for 4 h in open air. Then, the reaction was monitored by GC analysis using 5-methyl-2-furaldehyde as an internal standard. Separation of 2,5-DFF from reaction solution: after the epinephelos solution was filtered and washed with ethyl acetate for three times, the organic solvents were evaporated and the crude product was purified by flash column chromatography (PE:EA=3:1) to give the desired product in 88 % yield (detected by 1H NMR). 1H NMR (400 MHz, CDCl3): delta 9.84 (s, 2H), 7.33 (s, 2H). 13C NMR (100 MHz, CDCl3): delta 179.34, 154.30, 119.43. |
| 84% |
With oxygen; In toluene; at 110℃; under 760.051 Torr; for 6.0h; |
5-HMF is reacted in presence of several catalysts to produce DFF, in presence of organic solvents for 6 hours with 1 atm 02. Results are mentioned in Table 3. Table 3 It appears then that good yield and conversion are obtained with the process of the present invention with several variations regarding to the surfactant compounds used to produce the mesostructured VPO catalysts. |
| 84% |
With oxygen; In toluene; at 110℃; under 760.051 Torr; for 6.0h; |
5-HMF is reacted in presence of several catalysts to produce DFF, in presence of organic solvents for 6 hours with 1 atm 02. Results are mentioned in Table 3. Table 3 It appears then that good yield and conversion are obtained with the process of the present invention with several variations regarding to the surfactant compounds used to produce the mesostructured VPO catalysts. |
| 83% |
With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; copper(l) chloride; In acetonitrile; at 20℃; under 750.075 Torr; for 24.0h; |
All experiments were carried out in one-necked flasks equipped with a condenser. The condenser was left open in experiments using air as the oxidant and equipped with a balloon containing pure O2 in experiments using O2 as the oxidant. In a general procedure, HMF (125 mg, 1 mmol), CuCl (10 mg, 0.1 mmol) and TEMPO (17 mg, 0.1 mmol) were dissolved in solvent (5 mL). The reaction mixture was stirred magnetically (450 rpm) for 24 h and the reaction volume subsequently adjusted. Samples were collected periodically and analyzed via HPLC. More details about the instrument setup and HPLC analysis are found in the Supporting information. |
| 78% |
With [bis(acetoxy)iodo]benzene; oxygen; acetic acid; In ethyl acetate; at 40℃; under 760.051 Torr; for 1.0h; |
All experiments were carried out in two-necked round bottomflasks initially purged with O2 and then fastened with an O2-filled balloon (1 atm). The flasks were placed in a temperature-controlledoil bath with magnetic stirring. Typically, appropriate amounts of the reactants 5-HMF, TEMPO-SBA-15, BAIB, and acetic acid (if used) were allowed to react in ethyl acetate solvent (10-15 mL). The reaction mixture was magnetically stirred for a certain reaction time,at a controlled temperature. After each run, the reaction mixture was filtered to separate the solid catalyst. The filtrate was dried under reduced pressure, dissolved in deionized (DI) water and then analyzed in high pressure liquid chromatography (HPLC). |
| 66% |
With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; [bis(acetoxy)iodo]benzene; acetic acid; In ethyl acetate; at 30℃; for 0.75h;Green chemistry;Catalytic behavior; |
As described above, because BAIB play the TEMPO radical, the amount of TEMPO can be significantly reduced catalytically at stoichiometric levels (stoichiometric) level. As it is shown in Table 1, the 5-HMF, by using the case where a small amount of TEMPO and BAIB chemical stoichiometric amount of 4 hours at room temperature to obtain the 2,5-DFF of 34% (Entry 2). The results indicate that under a mild condition with addition BAIB, TEMPO or even promote the 5-HMF oxide in limited circumstances. This indicates that TEMPO radical that is being played by the BAIB. Referring to Figure 2 of the (B) HPLC chromatogram, CH-2,5 DFF with a product 3 is COOH is observed. These results are consistent with the reaction mechanism (see Fig. 3) described above. The CH 3 COOH may be due to the ligand exchange reaction of the by-product that is released during playback or TEMPO radical (see above reaction mechanism 2 (B)), BAIB (see reaction mechanism 2 (A)). The results described in the above means that the amount of TEMPO in the presence of BAIB will be significantly reduced. |
| 58% |
In 1,3,5-trimethyl-benzene; at 150℃; for 12.0h;Autoclave; Inert atmosphere; |
Add 5-hydroxymethylfurfural (5mmol), mesitylene (15mL), and reduced 6.6wt% Cu/gamma-Al2O3 catalyst (1.0g) to the autoclave. Pass N2 to exhaust the air in the empty reactor, stop the reaction after mechanically stirring the reaction at 150 C for 12h. The reaction was cooled to room temperature, the supernatant was centrifuged, and the catalyst was settled at the bottom. The supernatant was directly analyzed by gas chromatography. The reaction result was that the conversion of 5-hydroxymethylfurfural was 58.0%, The yield of formaldehyde was 36.6%. Wherein the detection of the product: After completion of the reaction, the reaction solution was centrifuged, the upper layer is a colored supernatant, the lower layer is a metal catalyst.The supernatant was centrifuged, filtered and analyzed by gas chromatography. |
| 46.7% |
With vanadium phosphate; In dimethyl sulfoxide; at 150℃; for 5.0h; |
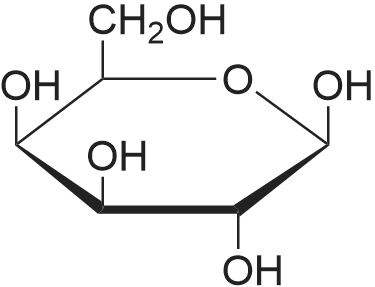
Example 61 mmol glucose and 0.3 mmol AlCl3 were placed in a round bottom flask. It was added 5mL of dimethyl sulfoxide. After stirring at room temperature for 10 minutes, heat to 120 deg. C. It is reacted at this temperature for 3h. Then,cool to room temperature. Thereto was added 0.1mmol VOPO4. After stirring evenly, heat to 150 deg.C. At this temperature, react for 5h. After completion of the reaction, 20mL of water and 30mL of methylene chloride was added, separated, the solvent was removed by distillation under reduced pressure, the product was purified by sublimation. The first step after the completion of the reaction part of the purified product was determined to HMF yield was 84.9%, the final DFF yield(based on glucose) of 46.7%. |
| 37.5% |
With oxygen; In N,N-dimethyl-formamide; under 760.051 Torr; for 4.0h;Heating; Green chemistry; |
The aerobic oxidation of HMF under atmospheric pressure wascarried out in a 25 mL round bottom flask, which was coupledwith a reflux condenser and capped with a balloon. Typically,HMF (1 mmol, 126 mg) was firstly dissolved into DMF (7 mL) witha magnetic stirrer. Then, the catalyst Fe3O4/Mn3O4was addedinto the reaction mixture and flushed with pure oxygen at a rateof 20 mL min-1. The reaction was carried out at 110C for thedesired reaction time. Time zero was taken when the oxygenwas flushed into the reaction mixture. After reaction, the cata-lyst Fe3O4/Mn3O4was separated from the reaction mixture bya permanent magnet, and the products were analyzed by HPLCmethod. |
| 7.9% |
With oxygen; In 1,4-dioxane; water; at 120℃; under 10343.2 Torr;Sealed tube; Inert atmosphere; |
[0235] Catalyst testing was conducted within 1 mL glass vials housed in a 96- well insert situated in a high pressure high throughput reactor. See Diamond, G. M., Murphy, V., Boussie, T. R., in Modern Applications of High Throughput R&D in Heterogeneous Catalysis, (eds, Hagemeyer, A. and Volpe, A. Jr. Bentham Science Publishers 2014, Chapter 8, 299-309); see also US 8,669,397, both of which are herein expressly incorporated by reference in their entireties. 10 mg of each powder catalyst was placed into a reactor along with 0.25 mL of a solution prepared in a 3:2 (wt/wt) dioxane:H20 mixture containing 0.5 M 5-hydroxymethylfurfural (HMF) (6.0 wt%). The 1 mL reaction vials within the insert were each covered with a Teflon sheet, a silicon mat and a steel gas diffusion plate each containing pin-holes to enable gas entry. The insert was placed within a pressure vessel which was leak tested under nitrogen pressure. The atmosphere within the reactor was then replaced by oxygen at a target pressure of 200 psig and the reactor was heated to a target temperature of 120C, and then shaken at 800 rpm for 120 min. After the reaction was completed, the shaking was stopped and the reactor was cooled down to room temperature. Samples were prepared for HPLC analysis by sampling from each reactor after diluting the sample with dimethyl sulfoxide (DMSO) and H2O. Reaction products were 5-hydroxymethylfurancarboxylic acid (HMFCA), 2,5- furandicarboxaldehyde (DFF), 5-formylfuran-3-carboxylic acid (FFCA) and 2,5- furandicarboxylic acid (FDCA). Each of the above products as well as remaining HMF were quantified through a calibration curve for each analyte by plotting the relative concentration against the relative detector response for calibration standards, and performing a fit to a parabolic expression. The mass balance (MB) is the sum of remaining HMF (not shown in Table 3), FFCA, and FDCA pathway products. The results are shown in Table 3. |
| 86%Chromat. |
With oxygen; acetic acid;cobalt(II) acetate; manganese(II) acetate; In butanone; at 120℃; under 51716.2 Torr; for 3.5h;Product distribution / selectivity; |
SELECTIVE OXIDATION OF HMF TO DFF USING Co/Mn CATALYSTS IN THE PRESENCE OF METHYL ETHYL KETONEExample 1[0032] A reaction mixture containing 97% purity HMF (5.0 g), acetic acid (50 mL), cobalt acetate (0.97 g), manganese acetate (0.98 g), and methyl ethyl ketone (1.90 mL) was placed in a 100 mL reactor and subjected to 1000 psi oxygen at 12O0C for 3.5 hours. The sample was spotted on TLC plates (K5F Whatman) and developed in 1 :1 EtOAc/hexane and visualized under UV light. Visual analysis indicated that after 3.5 hours, substantially all of the HMF was converted. The reaction mixture (58.58 g) was found to contain 46,356g/kg DFF (86%), 2,908 g/kg FFCA (5%), 4,201 g/kg HMF (8%) and 62 g/kg FDCA (1%) for a DFF selectivity of 86%. Subsequent GC/MS data revealed the conversion of HMF to DFF m/z = 124. Thus, after 3.5 hours, the conversion of HMF to DFF was essentially complete.. |
| 12.4%Chromat. |
With ammonium cerium (IV) nitrate; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; tetraethylammonium bromide; oxygen; sodium nitrite; In water; at 80℃; under 2250.23 Torr; for 3.0h;Autoclave; Sealed tube; |
General procedure: All oxidation experiments are performed in a 120mL autoclave equipped with the magnetic stirring and temperature control. A typical procedure for the oxidation of benzyl alcohol is as follows: 1.08g (10.0mmol) of benzyl alcohol, 0.0156g (0.1mmol) of TEMPO, 0.274g (0.5mmol) of CAN, 0.0690g (1.0mmol) of NaNO2, additive in suitable amount and 10mL of H2O were charged into the reactor, and the atmosphere inside is replaced with oxygen after the reactor is sealed. Then, pure oxygen is charged to 0.3MPa at room temperature. In the following, the autoclave is heated to 80C under stirring, and then kept for 2h. After reaction, the autoclave was cooled to room temperature and excess gas was purged. The mixture was transferred into a flask, in which the reactor was washed with CH2Cl2 for 3-5 times in order to transfer completely. Next, the products are extracted with 6mL CH2Cl2 three times. The obtained products were analyzed with internal standard technique by GC with a flame ionization detector (all products were determined on GC-MS with an Agilent 6890N GC/5973 MS detector). |
|
With vanadium(IV) oxide sulfate hydrate; copper(II) nitrate trihydrate; oxygen; In acetonitrile; at 80℃; under 750.075 Torr; for 1.5h;Autoclave;Activation energy; |
The aerobic oxidation of HMF was carried out in a 50-mL stain-less steel autoclave equipped with a magnetic stirrer, a pressure gauge and automatic temperature control apparatus. Typically, VOSO4(0.2 mmol, 34.3 mg), Cu(NO3)2·3H2O (0.2 mmol, 48.3 mg) and HMF (10 mmol, 1.26 g) were put into the autoclave with Teflon lining, followed by 5 mL acetonitrile. After the autoclave was closed, oxygen was added (0.1 MPa). The autoclave was then heated to 80 C within ca. 20 min. The reaction temperature was maintained at 80 C for 1.5 h. Oxygen was recharged if consumed during the oxidation. The autoclave was cooled to room temperature and depressurized carefully. A sample of the reaction mixture was taken for GC analysis, which were conducted on Agilent GC 7890D equipped with 19095J-323 capillary column(30 m × 530 m × 1.5 m) and a flame ionization detector. The quantitative results were based on the internal standard method using mesitylene as internal standard. The results reported as conversion and selectivity are expressed in mol% based on the total HMF intake. |
|
With (x)H2O*KMn8O16; In N,N-dimethyl-formamide; at 109.84℃; for 1.0h;Autoclave; High pressure; |
General procedure: HMF oxidation reactions were carried out in a Teflon-lined stainless steel autoclave (50 mL). Typically, 1 mmol HMF (98%, Alfa Aesar) and 50 mg catalysts were introduced to 10 mL DMF (J.T.Baker, 60.02% H2O) in the autoclave and were stirred at ca.700 rpm. The reactants and products were analyzed by HPLC (ShimadzuLC-20A) using a UV detector and an Alltech OA-1000 organic acid column (0.005 M H2SO4 mobile phase, 0.7 mL/minflow rate, and 353 K oven temperature). HMF reaction activities were reported as molar HMF conversion rates per gram (or m2) of catalysts per hour (i.e., mmol HMF/(gcat h) or mmol HMF/(m2cat h)) and selectivities on a carbon basis. For catalyst recycling tests, if not specially stated, the retrieved catalysts were washed thoroughly with deionized water and then dried in vacuum oven before being recycled. |
|
With 4-acetylamino-2,2,6,6-tetramethyl-1-piperidinoxy; nitric acid; acetic acid;Autoclave;Catalytic behavior; |
10 g (79 mmol) of 5-(hydroxymethyl)furfural (referred to herein as 5-HMF, purity of 98%), 0.213 g (1 mmol) of 4-acetoamino(2,2,6,6-tetramethylpiperid-1-yl)oxyl (referred to herein as 4AA Tempo), 100 g (1.67 mol) of acetic acid and 4.35 g of nitric acid at 0.91 mol/1 (4 mmol) are placed in a stainless-steel autoclave with an internal volume of 600 ml, sold by the company Parr (model No. 4346), equipped with a variable-speed stirrer and a double-paddle system in the form of an impeller, a gas inlet tube connected to a pressurized bottle equipped with a pressure regulator, a gas evacuation tube, a cooling coil and a system for measuring and regulating the temperature. The ratios are established so as to have 2.1% by weight of 4AA Tempo, 5 mol % of nitric acid relative to the 5-HMF. The HNO3/4AA Tempo ratio is thus 4. Once all the reagents have been placed in the autoclave, it is purged once at 0.3 MPa with oxygen and then heated under 0.1 MPa of oxygen. The stirring speed is then adjusted to 1600 rpm. When the reactor reaches 70 C., the oxygen pressure is adjusted to 0.3 MPa. The consumption of oxygen starts at 80 C. The temperature is regulated at 85 C. for 1 hour. After one hour of contact time, the autoclave is cooled by simply switching off the heating, with removal of the heating mantle, without circulation of water in the cooling loop (which avoids crystallization of the DFF on the coil). The stirring is lowered to 250 rpm during the cooling. When the reactor reaches 60 C., the stirring is stopped and the pressure is reduced to atmospheric pressure. The autoclave is opened at 60 C., all the components are in solution, and there are neither any crystals nor any precipitates. A sample of the crude reaction product is taken and analyzed by gas chromatography (GC) and the results are expressed as a percentage of the area distribution. The composition of the crude reaction product is presented in Table 1. |
|
With oxygen; In N,N-dimethyl-formamide; at 120℃; under 760.051 Torr; for 8.0h;Catalytic behavior; |
2.4. General procedure for the oxidation of HMF HMF (1 mmol), DMF (3 mL), and catalyst (20 mg) were mixed in athree-neck ask. Then the mixture was heated to 120 C and stirredunder O2 atmosphere for 8 h at atmospheric pressure. O2 was chargedfrom a balloon. After the reaction was completed, the catalyst was l-tered to be used in the next cycle. The conversion and selectivity are de-termined by HPLC analysis. |
|
With ruthenium-carbon composite; oxygen; In toluene; at 110℃; under 14997.7 Torr; for 1.0h; |
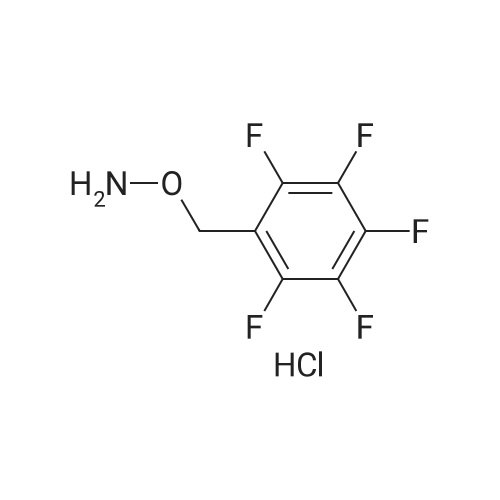
[0035] In a second process, a mixture of 5-hydroxymethylfurfural (1 equivalent), ruthenium on activated carbon catalyst (0.01 equivalent metal) and toluene as solvent is treated with oxygen gas (about 290 psi) while heating at about 110 C for about 1 hour. After filtration to remove the catalyst the toluene is removed by evaporation under reduced pressure to yield 2,5-diformylfuran. A mixture of 2,5-diformylfuran (1 equivalent), hydroxylamine hydrochloride (2 equivalents), potassium acetate (2 equivalents) and 50% aqueous ethanol is heated at about 50 C for about 1 hour. The precipitate is filtered, washed with water and dried under reduced pressure to yield 2,5-diformylfuran dioxime. A mixture of 2,5-diformylfuran dioxime (1 equivalent), Raney nickel (about 5 grams per mmol of dioxime) and tetrahydrofuran as solvent is treated with hydrogen gas (about 50 bar) in an autoclave. When no more hydrogen is absorbed, the catalyst is removed by filtration under argon gas and rinsed with tetrahydrofuran. The combined filtrates are concentrated under reduced pressure to yield 2,5-bis(aminomethyl)furan that is purified by recrystallization of its dihydrobromide salt. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping