Alternatived Products of [ 56221-42-2 ]
Product Details of [ 56221-42-2 ]
| CAS No. : | 56221-42-2 |
MDL No. : | MFCD08437123 |
| Formula : |
C11H10O4
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | - |
| M.W : |
206.20
|
Pubchem ID : | - |
| Synonyms : |
|
Safety of [ 56221-42-2 ]
| Signal Word: | |
Class: | |
| Precautionary Statements: | |
UN#: | |
| Hazard Statements: | |
Packing Group: | |
Application In Synthesis of [ 56221-42-2 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 56221-42-2 ]
- 1
-
 [ 326-56-7 ]
[ 326-56-7 ]

-
 [ 56221-42-2 ]
[ 56221-42-2 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With diethyl ether; sodium; acetone |
|
- 2
-
 [ 4433-92-5 ]
[ 4433-92-5 ]

-
 [ 56221-42-2 ]
[ 56221-42-2 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With ethanol |
|
| Yield | Reaction Conditions | Operation in experiment |
|
Rk. m. FeCl3, A. --> kirschrote Farbe; |
|
| Yield | Reaction Conditions | Operation in experiment |
|
4-Piperonoyl-acetessigsaeure, A., Kochen; |
|
|
3,4-Methylendioxy-1-<5,5-dichlor-3-oxo-penten-(4)-in-(1)-yl>benzol, HCl, Eg., neben 3,4-Methylendioxy-acetophenon; |
|
|
3,5-Dioxo-5-piperonyl-pentansaeure, wss. A., Erwaermen; |
|
- 5
-
 [ 4433-92-5 ]
[ 4433-92-5 ]

-
 [ CAS Unavailable ]
[ CAS Unavailable ]

-
 [ 56221-42-2 ]
[ 56221-42-2 ]
- 6
-
 [ 1027191-19-0 ]
[ 1027191-19-0 ]

-
 [ 56221-42-2 ]
[ 56221-42-2 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With copper(II) sulfate In methanol; water at 60℃; for 1.5h; |
|
- 7
-
 [ 37673-10-2 ]
[ 37673-10-2 ]

-
 [ 56221-42-2 ]
[ 56221-42-2 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: NaH / dimethylformamide / 1 h / 27 - 30 °C
2: CuSO4*5H2O / methanol; H2O / 1.5 h / 60 °C |
|
- 8
-
 [ 6969-80-8 ]
[ 6969-80-8 ]

-
 [ 56221-42-2 ]
[ 56221-42-2 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: ethanolic KOH-solution |
|
- 9
-
 [ 56221-42-2 ]
[ 56221-42-2 ]

-
 [ 1821300-57-5 ]
[ 1821300-57-5 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 3 steps
1: triethylamine / ethanol / 48 h / 20 °C
2: sodium ethanolate / ethanol / 2 h / 50 °C
3: sodium hydride / mineral oil; N,N-dimethyl-formamide / 48 h / 20 °C |
|
Reference:
[1]Salerno, Loredana; Modica, Maria N.; Romeo, Giuseppe; Pittalà, Valeria; Cagnotto, Alfredo; Siracusa, Maria A.
[Medicinal Chemistry, 2015, vol. 11, # 2, p. 109 - 117]
- 10
-
 [ 56221-42-2 ]
[ 56221-42-2 ]

-
 [ 1821300-58-6 ]
[ 1821300-58-6 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 3 steps
1: triethylamine / ethanol / 48 h / 20 °C
2: sodium ethanolate / ethanol / 2 h / 50 °C
3: sodium hydride / mineral oil; N,N-dimethyl-formamide / 48 h / 20 °C |
|
Reference:
[1]Salerno, Loredana; Modica, Maria N.; Romeo, Giuseppe; Pittalà, Valeria; Cagnotto, Alfredo; Siracusa, Maria A.
[Medicinal Chemistry, 2015, vol. 11, # 2, p. 109 - 117]
- 11
-
 [ 56221-42-2 ]
[ 56221-42-2 ]

-
 [ 1821300-59-7 ]
[ 1821300-59-7 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 3 steps
1: triethylamine / ethanol / 48 h / 20 °C
2: sodium ethanolate / ethanol / 2 h / 50 °C
3: sodium hydride / mineral oil; N,N-dimethyl-formamide / 48 h / 20 °C |
|
Reference:
[1]Salerno, Loredana; Modica, Maria N.; Romeo, Giuseppe; Pittalà, Valeria; Cagnotto, Alfredo; Siracusa, Maria A.
[Medicinal Chemistry, 2015, vol. 11, # 2, p. 109 - 117]
- 12
-
 [ 56221-42-2 ]
[ 56221-42-2 ]

-
 [ 1821300-60-0 ]
[ 1821300-60-0 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 3 steps
1: triethylamine / ethanol / 48 h / 20 °C
2: sodium ethanolate / ethanol / 2 h / 50 °C
3: sodium hydride / mineral oil; N,N-dimethyl-formamide / 48 h / 20 °C |
|
Reference:
[1]Salerno, Loredana; Modica, Maria N.; Romeo, Giuseppe; Pittalà, Valeria; Cagnotto, Alfredo; Siracusa, Maria A.
[Medicinal Chemistry, 2015, vol. 11, # 2, p. 109 - 117]
- 13
-
 [ 56221-42-2 ]
[ 56221-42-2 ]

-
 [ 1821300-61-1 ]
[ 1821300-61-1 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 3 steps
1: triethylamine / ethanol / 48 h / 20 °C
2: sodium ethanolate / ethanol / 2 h / 50 °C
3: sodium hydride / mineral oil; N,N-dimethyl-formamide / 48 h / 20 °C |
|
Reference:
[1]Salerno, Loredana; Modica, Maria N.; Romeo, Giuseppe; Pittalà, Valeria; Cagnotto, Alfredo; Siracusa, Maria A.
[Medicinal Chemistry, 2015, vol. 11, # 2, p. 109 - 117]
- 14
-
 [ 56221-42-2 ]
[ 56221-42-2 ]

-
 [ 1821300-62-2 ]
[ 1821300-62-2 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 3 steps
1: triethylamine / ethanol / 48 h / 20 °C
2: sodium ethanolate / ethanol / 2 h / 50 °C
3: sodium hydride / mineral oil; N,N-dimethyl-formamide / 48 h / 20 °C |
|
Reference:
[1]Salerno, Loredana; Modica, Maria N.; Romeo, Giuseppe; Pittalà, Valeria; Cagnotto, Alfredo; Siracusa, Maria A.
[Medicinal Chemistry, 2015, vol. 11, # 2, p. 109 - 117]
- 15
-
 [ 56221-42-2 ]
[ 56221-42-2 ]

-
 [ CAS Unavailable ]
[ CAS Unavailable ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 3 steps
1: triethylamine / ethanol / 48 h / 20 °C
2: sodium ethanolate / ethanol / 2 h / 50 °C
3: sodium hydride / mineral oil; N,N-dimethyl-formamide / 48 h / 20 °C |
|
Reference:
[1]Salerno, Loredana; Modica, Maria N.; Romeo, Giuseppe; Pittalà, Valeria; Cagnotto, Alfredo; Siracusa, Maria A.
[Medicinal Chemistry, 2015, vol. 11, # 2, p. 109 - 117]
- 16
-
 [ 56221-42-2 ]
[ 56221-42-2 ]

-
 [ 1821300-71-3 ]
[ 1821300-71-3 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 4 steps
1: triethylamine / ethanol / 48 h / 20 °C
2: sodium ethanolate / ethanol / 2 h / 50 °C
3: sodium hydride / mineral oil; N,N-dimethyl-formamide / 48 h / 20 °C
4: potassium hydroxide; water / ethanol / 2 h / Reflux |
|
Reference:
[1]Salerno, Loredana; Modica, Maria N.; Romeo, Giuseppe; Pittalà, Valeria; Cagnotto, Alfredo; Siracusa, Maria A.
[Medicinal Chemistry, 2015, vol. 11, # 2, p. 109 - 117]
- 17
-
 [ 56221-42-2 ]
[ 56221-42-2 ]

-
 [ 1821300-73-5 ]
[ 1821300-73-5 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 4 steps
1: triethylamine / ethanol / 48 h / 20 °C
2: sodium ethanolate / ethanol / 2 h / 50 °C
3: sodium hydride / mineral oil; N,N-dimethyl-formamide / 48 h / 20 °C
4: potassium hydroxide; water / ethanol / 2 h / Reflux |
|
Reference:
[1]Salerno, Loredana; Modica, Maria N.; Romeo, Giuseppe; Pittalà, Valeria; Cagnotto, Alfredo; Siracusa, Maria A.
[Medicinal Chemistry, 2015, vol. 11, # 2, p. 109 - 117]
- 18
-
 [ 56221-42-2 ]
[ 56221-42-2 ]

-
 [ CAS Unavailable ]
[ CAS Unavailable ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 4 steps
1: triethylamine / ethanol / 48 h / 20 °C
2: sodium ethanolate / ethanol / 2 h / 50 °C
3: sodium hydride / mineral oil; N,N-dimethyl-formamide / 48 h / 20 °C
4: potassium hydroxide; water / ethanol / 2 h / Reflux |
|
Reference:
[1]Salerno, Loredana; Modica, Maria N.; Romeo, Giuseppe; Pittalà, Valeria; Cagnotto, Alfredo; Siracusa, Maria A.
[Medicinal Chemistry, 2015, vol. 11, # 2, p. 109 - 117]
- 19
-
 [ 56221-42-2 ]
[ 56221-42-2 ]

-
 [ 1821300-77-9 ]
[ 1821300-77-9 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 4 steps
1: triethylamine / ethanol / 48 h / 20 °C
2: sodium ethanolate / ethanol / 2 h / 50 °C
3: sodium hydride / mineral oil; N,N-dimethyl-formamide / 48 h / 20 °C
4: potassium hydroxide; water / ethanol / 2 h / Reflux |
|
Reference:
[1]Salerno, Loredana; Modica, Maria N.; Romeo, Giuseppe; Pittalà, Valeria; Cagnotto, Alfredo; Siracusa, Maria A.
[Medicinal Chemistry, 2015, vol. 11, # 2, p. 109 - 117]
- 20
-
 [ 56221-42-2 ]
[ 56221-42-2 ]

-
 [ 1821300-79-1 ]
[ 1821300-79-1 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 4 steps
1: triethylamine / ethanol / 48 h / 20 °C
2: sodium ethanolate / ethanol / 2 h / 50 °C
3: sodium hydride / mineral oil; N,N-dimethyl-formamide / 48 h / 20 °C
4: potassium hydroxide; water / ethanol / 2 h / Reflux |
|
Reference:
[1]Salerno, Loredana; Modica, Maria N.; Romeo, Giuseppe; Pittalà, Valeria; Cagnotto, Alfredo; Siracusa, Maria A.
[Medicinal Chemistry, 2015, vol. 11, # 2, p. 109 - 117]
- 21
-
 [ 56221-42-2 ]
[ 56221-42-2 ]

-
 [ 1821300-81-5 ]
[ 1821300-81-5 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 4 steps
1: triethylamine / ethanol / 48 h / 20 °C
2: sodium ethanolate / ethanol / 2 h / 50 °C
3: sodium hydride / mineral oil; N,N-dimethyl-formamide / 48 h / 20 °C
4: potassium hydroxide; water / ethanol / 2 h / Reflux |
|
Reference:
[1]Salerno, Loredana; Modica, Maria N.; Romeo, Giuseppe; Pittalà, Valeria; Cagnotto, Alfredo; Siracusa, Maria A.
[Medicinal Chemistry, 2015, vol. 11, # 2, p. 109 - 117]
- 22
-
 [ 56221-42-2 ]
[ 56221-42-2 ]

-
 [ 1821300-82-6 ]
[ 1821300-82-6 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 4 steps
1: triethylamine / ethanol / 48 h / 20 °C
2: sodium ethanolate / ethanol / 2 h / 50 °C
3: sodium hydride / mineral oil; N,N-dimethyl-formamide / 48 h / 20 °C
4: potassium hydroxide; water / ethanol / 2 h / Reflux |
|
Reference:
[1]Salerno, Loredana; Modica, Maria N.; Romeo, Giuseppe; Pittalà, Valeria; Cagnotto, Alfredo; Siracusa, Maria A.
[Medicinal Chemistry, 2015, vol. 11, # 2, p. 109 - 117]
- 23
-
 [ 56221-42-2 ]
[ 56221-42-2 ]

-
 [ 1821300-50-8 ]
[ 1821300-50-8 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: triethylamine / ethanol / 48 h / 20 °C
2: sodium ethanolate / ethanol / 2 h / 50 °C |
|
Reference:
[1]Salerno, Loredana; Modica, Maria N.; Romeo, Giuseppe; Pittalà, Valeria; Cagnotto, Alfredo; Siracusa, Maria A.
[Medicinal Chemistry, 2015, vol. 11, # 2, p. 109 - 117]
- 24
-
 [ 56221-42-2 ]
[ 56221-42-2 ]

-
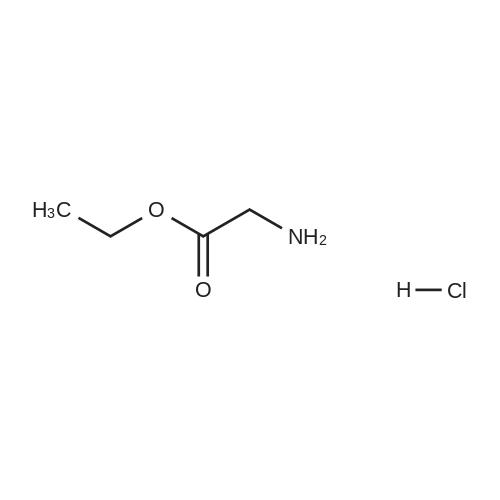 [ 623-33-6 ]
[ 623-33-6 ]

-
 [ 1821300-49-5 ]
[ 1821300-49-5 ]
| Yield | Reaction Conditions | Operation in experiment |
| 75% |
With triethylamine In ethanol at 20℃; for 48h; |
General Procedure for the Synthesis of Ethyl-N-(3-oxo-1-alkenyl)glycinates 5-8
General procedure: Glycine ethyl ester hydrochloride (24 mmol) and triethylamine(24 mmol) were added dropwise to an ethanolic solution(30 mL) of 20 mmol of the appropriate derivative 1-4,purchased (1) or synthesized (2-4) as described in literature[20-22]. The mixture was stirred at room temperature for 48hours. After this time, water was added and the obtainedsolid was filtered and washed with water to give correspondingethyl-N-(3-oxo-1-alkenyl)glycinate (5-8) as solid. Usingthis procedure the following compounds were obtained: |
Reference:
[1]Salerno, Loredana; Modica, Maria N.; Romeo, Giuseppe; Pittalà, Valeria; Cagnotto, Alfredo; Siracusa, Maria A.
[Medicinal Chemistry, 2015, vol. 11, # 2, p. 109 - 117]
- 25
-
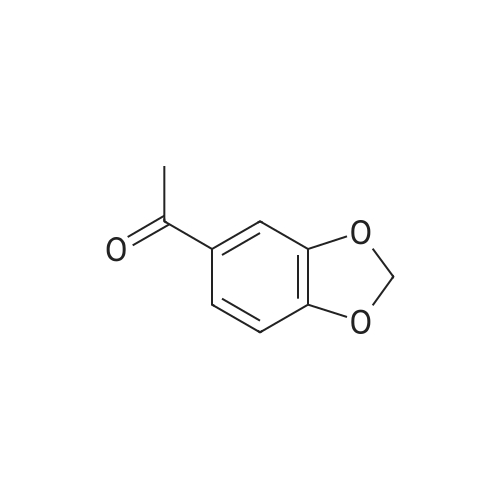 [ 3162-29-6 ]
[ 3162-29-6 ]

-
 [ 141-78-6 ]
[ 141-78-6 ]

-
 [ 56221-42-2 ]
[ 56221-42-2 ]
| Yield | Reaction Conditions | Operation in experiment |
| 56% |
With sodium hydride at 0 - 20℃; for 12h; |
|
|
Stage #1: 1-(benzo[d][1,3]dioxol-6-yl)ethanone With sodium hydride In tetrahydrofuran at 0℃; Inert atmosphere;
Stage #2: ethyl acetate In tetrahydrofuran at 20℃; for 12h; Inert atmosphere; |
4.1.1 4.1.1. The synthesis of N-hydroxy-4-(5-(p-tolyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzamide (8)
General procedure: A solution of 27 p-methylacetophenone (0.67g, 5mmol) in dry 28 THF (20mL) was added dropwise to 29 NaH (0.24g, 10mmol) in dry THF (50mL) under nitrogen atomosphere and the mixture was stirred for 1h at 0°C. Then a solution of 30 ethyl trifluoroacetate (0.9mL, 7.5mmol) in dry THF (10mL) was added dropwise. The mixture was warmed to room temperature for 12h. The reaction was quenched with saturation NaHCO3 and extracted with ethyl acetate (50mL×3). The combined organic extracts were washed with brine (50mL), dried with Na2SO4 and evaporated. Finally, the resulting residue was purified by column chromatography on silica gel as indicated to give 6a as canary yellow solid. |
Reference:
[1]An, Zhenyu; Liu, Yafeng; Yan, Rulong; Zhao, Pengbo
[Advanced Synthesis and Catalysis, 2021, vol. 363, # 13, p. 3240 - 3244]
[2]Yang, Jinyu; Cheng, Gaoliang; Xu, Qihao; Luan, Shenglin; Wang, Shuxiang; Liu, Dan; Zhao, Linxiang
[Bioorganic and Medicinal Chemistry, 2018, vol. 26, # 8, p. 1418 - 1425]
- 26
-
 [ 56221-42-2 ]
[ 56221-42-2 ]

-
 [ 2226206-23-9 ]
[ 2226206-23-9 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: acetic acid; hydrazine hydrate / 80 °C
2: acetonitrile / 70 °C / Alkaline conditions |
|
Reference:
[1]Yang, Jinyu; Cheng, Gaoliang; Xu, Qihao; Luan, Shenglin; Wang, Shuxiang; Liu, Dan; Zhao, Linxiang
[Bioorganic and Medicinal Chemistry, 2018, vol. 26, # 8, p. 1418 - 1425]
- 27
-
 [ 56221-42-2 ]
[ 56221-42-2 ]

-
 [ 2226196-09-2 ]
[ 2226196-09-2 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: acetic acid; hydrazine hydrate / 80 °C
2: acetonitrile / 70 °C / Alkaline conditions |
|
Reference:
[1]Yang, Jinyu; Cheng, Gaoliang; Xu, Qihao; Luan, Shenglin; Wang, Shuxiang; Liu, Dan; Zhao, Linxiang
[Bioorganic and Medicinal Chemistry, 2018, vol. 26, # 8, p. 1418 - 1425]

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping







