Alternatived Products of [ 55748-84-0 ]
Product Details of [ 55748-84-0 ]
| CAS No. : | 55748-84-0 |
MDL No. : | MFCD08437203 |
| Formula : |
C10H8Cl2O2
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | - |
| M.W : |
231.08
|
Pubchem ID : | - |
| Synonyms : |
|
Safety of [ 55748-84-0 ]
| Signal Word: | |
Class: | |
| Precautionary Statements: | |
UN#: | |
| Hazard Statements: | |
Packing Group: | |
Application In Synthesis of [ 55748-84-0 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 55748-84-0 ]
- 1
-
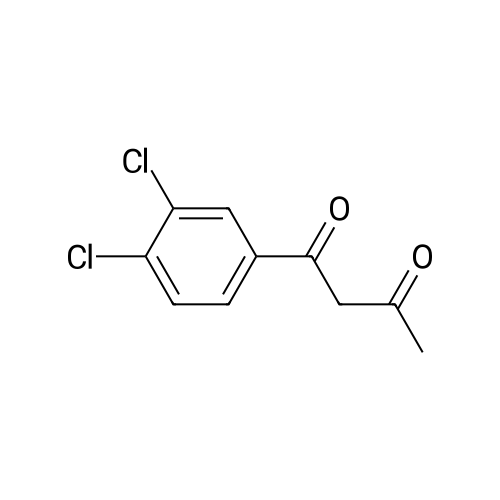 [ 55748-84-0 ]
[ 55748-84-0 ]

-
 [ CAS Unavailable ]
[ CAS Unavailable ]
| Yield | Reaction Conditions | Operation in experiment |
|
With bromine In chloroform for 24h; |
|
- 2
-
 [ CAS Unavailable ]
[ CAS Unavailable ]

-
 [ 55748-84-0 ]
[ 55748-84-0 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With copper(II) sulfate In methanol; water at 60℃; for 1.5h; |
|
- 3
-
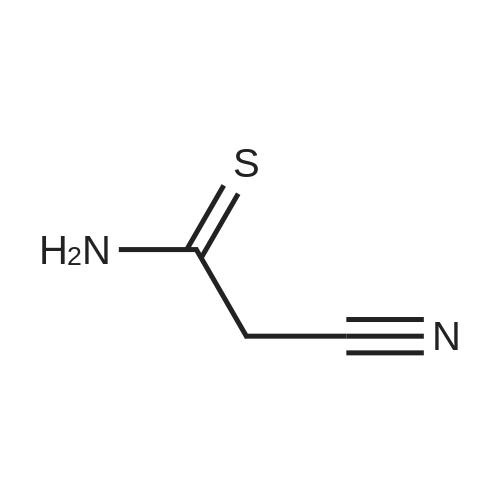 [ 7357-70-2 ]
[ 7357-70-2 ]

-
 [ 55748-84-0 ]
[ 55748-84-0 ]

-
 [ CAS Unavailable ]
[ CAS Unavailable ]
| Yield | Reaction Conditions | Operation in experiment |
|
With 1,4-diaza-bicyclo[2.2.2]octane In ethanol at 20℃; Reflux; |
Step c
General procedure: Cyanothioacetamide (for X=S) (1.5 equiv) or cyanoacetamide (for X=O) (1.5 equiv) was added to a solution of the 1, 3-diones (for R4= -CF3 and -CH3) (1.0 equiv) or enaminones (for R4=H) (1.0 equiv) in ethanol in the presence of DABCO (1.0 equiv) at room temperature. The reaction mixture was stirred under reux for 3-6 h until complete conversion of the starting materials, as monitored by TLC. After cooled to room temperature, the solvent was evaporated under reduced pressure and the residue was neutralized with diluted hydrochloric acid (1 N) to precipitate the crude products. After filtrated and dried in vacuo, the product can be straight used for step d. Yield: 70-90%. |
Reference:
[1]Wang, Ning-Yu; Zuo, Wei-Qiong; Xu, Ying; Gao, Chao; Zeng, Xiu-Xiu; Zhang, Li-Dan; You, Xin-Yu; Peng, Cui-Ting; Shen, Yang; Yang, Sheng-Yong; Wei, Yu-Quan; Yu, Luo-Ting
[Bioorganic and Medicinal Chemistry Letters, 2014, vol. 24, # 6, p. 1581 - 1588]
- 4
-
 [ 55748-84-0 ]
[ 55748-84-0 ]

-
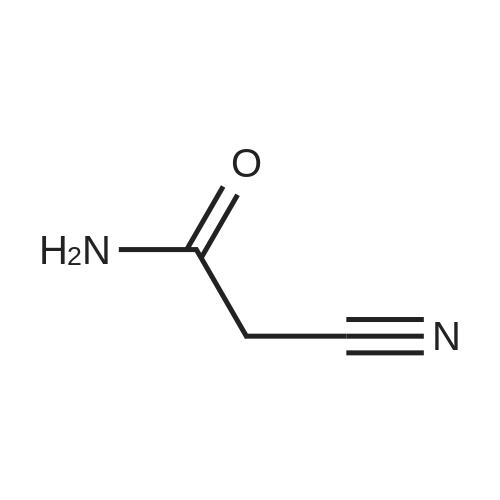 [ 107-91-5 ]
[ 107-91-5 ]

-
 [ 235100-60-4 ]
[ 235100-60-4 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With 1,4-diaza-bicyclo[2.2.2]octane In ethanol at 20℃; Reflux; |
Step c
General procedure: Cyanothioacetamide (for X=S) (1.5 equiv) or cyanoacetamide (for X=O) (1.5 equiv) was added to a solution of the 1, 3-diones (for R4= -CF3 and -CH3) (1.0 equiv) or enaminones (for R4=H) (1.0 equiv) in ethanol in the presence of DABCO (1.0 equiv) at room temperature. The reaction mixture was stirred under reux for 3-6 h until complete conversion of the starting materials, as monitored by TLC. After cooled to room temperature, the solvent was evaporated under reduced pressure and the residue was neutralized with diluted hydrochloric acid (1 N) to precipitate the crude products. After filtrated and dried in vacuo, the product can be straight used for step d. Yield: 70-90%. |
Reference:
[1]Wang, Ning-Yu; Zuo, Wei-Qiong; Xu, Ying; Gao, Chao; Zeng, Xiu-Xiu; Zhang, Li-Dan; You, Xin-Yu; Peng, Cui-Ting; Shen, Yang; Yang, Sheng-Yong; Wei, Yu-Quan; Yu, Luo-Ting
[Bioorganic and Medicinal Chemistry Letters, 2014, vol. 24, # 6, p. 1581 - 1588]
- 5
-
 [ 141-78-6 ]
[ 141-78-6 ]

-
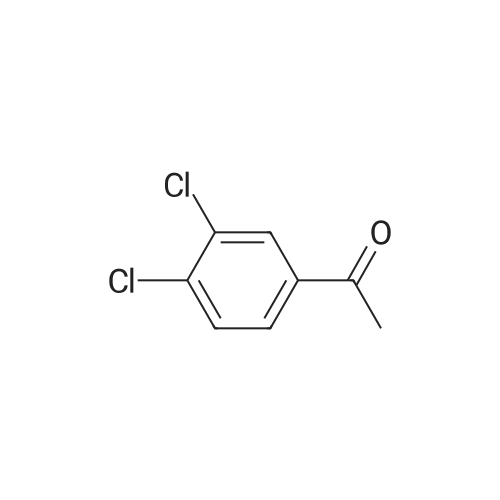 [ 2642-63-9 ]
[ 2642-63-9 ]

-
 [ 55748-84-0 ]
[ 55748-84-0 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With sodium methylate In tetrahydrofuran; methanol at 0 - 20℃; |
Step a
General procedure: To a freshly prepared sodium methylate solution in methanol and THF ethyl trifluoroacetate (1.2 equiv) was added under stirring at 0° followed by addition of ketone 2 (1.0 equiv). The reaction mixture was allowed to stir for additional 3-24 h until the starting materials were consumed, as determined by thin-layer chromatography (TLC). Then the solvent was removed under reduced pressure and the residue was acidified with hydrochloric acid (1 N), followed by extracted with acetic ether. The combined organic layers were dried (MgSO4), Fitered and the filtrate was concentrated under reduced pressure. The crude product was puried by column chromatography. Yield: 40-90%. For some cases, the crude products can be straight used for step c without the column chromatography procedure. |
|
With sodium hydride In diethyl ether at -5 - 25℃; |
3.2.2. General Synthetic Procedure of Intermediate III
General procedure: Two different procedures were used to synthesize 1,3-diketones in this study. (1) When R1 was a methyl group, ethyl acetate was used as the reactant and solvent. In a 100 mLround-bottom ask, 60% sodium hydride (28.8 mmol) was slowly added to 30 mL of ethyl acetate under stirring at 5 °C. There after, a mixture of methyl ketones (28.8 mmol) and10 mL of ethyl acetate was added slowly to the reaction solution. After addition, the entire system was warmed to room temperature and stirred for 6 h. The reaction was quenched with aqueous hydrochloric acid (1 N, 30 mL), acidified to a pH range of 1-2, and subsequently extracted using ethyl acetate (3 15 mL). The combined organic phases were dried over anhydrous sodium sulfate and concentrated under a vacuum. The residue was purified via flash column chromatography (n-hexane) to obtain intermediate III (yields 65.8-78.4%). (2) When R1 was a difluoromethyl or trifluoromethyl, the procedure was as follows: in a 100 mL round-bottom flask, 60% sodium hydride (43.2 mmol) was slowly added to 30 mL of ethyl ether with stirring at 5 °C. Thereafter, a mixture of methylketones (28.8 mmol) and ester (34.56 mmol) was slowly added to the reaction solution.After addition, the entire system was warmed to room temperature and stirred overnight.The reaction was quenched with aqueous hydrochloric acid (1 N, 30 mL), acidified to a pHrange of 1±2, and extracted using ethyl acetate (3 15 mL). The combined organic phases were dried over anhydrous sodium sulfate and concentrated under vacuum. The residue was puried via ash column chromatography (n-hexane/ethyl acetate = 10:1) to afford intermediate III (yields 87.6-92.2%). |
Reference:
[1]Wang, Ning-Yu; Zuo, Wei-Qiong; Xu, Ying; Gao, Chao; Zeng, Xiu-Xiu; Zhang, Li-Dan; You, Xin-Yu; Peng, Cui-Ting; Shen, Yang; Yang, Sheng-Yong; Wei, Yu-Quan; Yu, Luo-Ting
[Bioorganic and Medicinal Chemistry Letters, 2014, vol. 24, # 6, p. 1581 - 1588]
[2]Feng, Tong; Liu, Qing; Xu, Zhi-Yuan; Li, Hui-Ting; Wei, Wei; Shi, Rong-Chuan; Zhang, Li; Cao, Yi-Ming; Liu, Shang-Zhong
[Molecules, 2023, vol. 28, # 3]
- 6
-
 [ 55748-84-0 ]
[ 55748-84-0 ]

-
 [ 762-04-9 ]
[ 762-04-9 ]

-
 [ 1691220-64-0 ]
[ 1691220-64-0 ]
| Yield | Reaction Conditions | Operation in experiment |
| 55% |
With dipotassium peroxodisulfate; silver(I) acetate In water; N,N-dimethyl-formamide at 85℃; for 24h; Inert atmosphere; Schlenk technique; chemoselective reaction; |
|
Reference:
[1]Li, Lili; Huang, Wenbin; Chen, Lijin; Dong, Jiaxing; Ma, Xuebing; Peng, Yungui
[Angewandte Chemie - International Edition, 2017, vol. 56, # 35, p. 10539 - 10544][Angew. Chem., 2017, vol. 129, # 35, p. 10675 - 10680,6]
- 7
-
 [ 55748-84-0 ]
[ 55748-84-0 ]

-
 [ CAS Unavailable ]
[ CAS Unavailable ]

-
 [ CAS Unavailable ]
[ CAS Unavailable ]
| Yield | Reaction Conditions | Operation in experiment |
|
With sulfuric acid In ethanol at 20 - 75℃; |
3.2.3. General Synthetic Procedure of Intermediate IV
General procedure: In a 50 mL round-bottom flask, intermediate III (5 mmol) was added to a solution of intermediate II (5 mmol) in 20 mL of ethanol at room temperature. There after, concentrated sulfuric acid was slowly added to the stirred solution, and the reaction mixture was heated to 75 °C in an oil bath and kept at reflux for 2 h. The reaction was cooled to room temperature,quenched with a saturated sodium carbonate solution, and extracted using ethyl acetate (3 15 mL). The combined organic phases were dried over anhydrous sodium sulfate and concentrated under vacuum. The residue was purified via flash column chromatography(n-hexane/ethyl acetate = 6:1) to afford intermediate IV (yields 80.3-91.7%). |
Reference:
[1]Feng, Tong; Liu, Qing; Xu, Zhi-Yuan; Li, Hui-Ting; Wei, Wei; Shi, Rong-Chuan; Zhang, Li; Cao, Yi-Ming; Liu, Shang-Zhong
[Molecules, 2023, vol. 28, # 3]

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping








