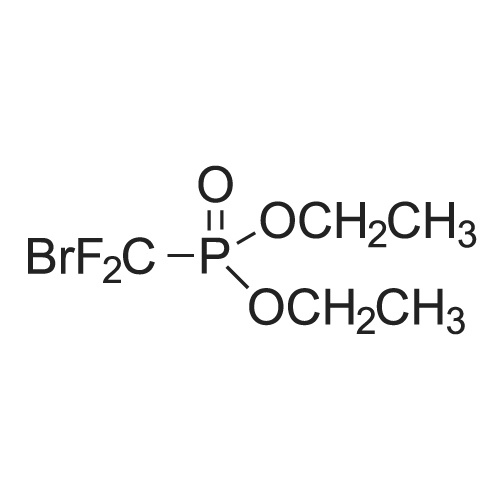
| 95% |
|
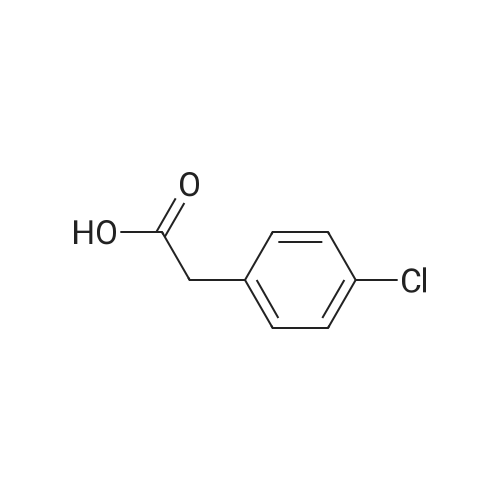
(101.8 mmol) of 4-chlorophenylacetic acid and 0.37 mg (0.46 mmol) of sodium benzoate were added to a 50 ml reaction flask equipped with a mechanical stirrer, thermometer, water separator and reflux condenser. The temperature was increased to 110 C, and 10 g (92.5 mmol) of o-phenylenediamine was added thereto, and the temperature was raised to 120 C for 1 hour. The reaction process and reaction endpoint were monitored by TLC (Developing solvent: ethyl acetate: petroleum ether = 1: 1, Rf = 0.41). The reactants were neutralized with 5 wt% Na0H solution and stirred until the pH was 7. 0 to 8.5; filter, washed with water to neutral crude, with ethanol - water mixed solvent recrystallization (volume ratio of 1: 2 ~ 1: 3), filtration, drying in pure 21 3g, the |
| 88.2% |
In toluene; at 110℃; for 6.08333h; |
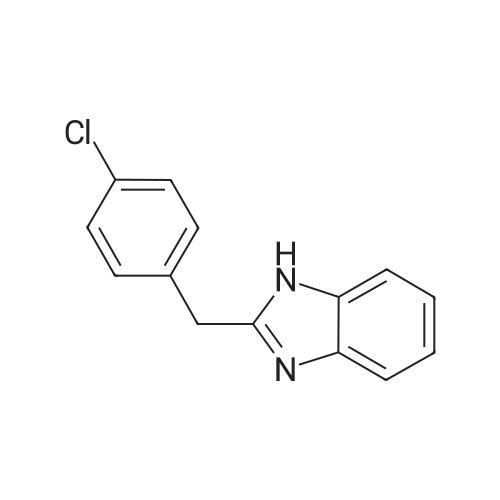
10.8 g of o-phenylenediamine and 20.4 g of 4-chlorophenylacetic acid were placed in a 250 ml three-neck round bottom flask.Add 120 ml of toluene, stir for 5 minutes, add 1 g of Zn/B2O3 catalyst, connect the water separator, and heat to reflux at 110 C for 6 hours.The solvent is recovered by using a distillation recovery device. When about 100 ml of toluene is recovered, the mixture is distilled under reduced pressure. When 110 ml of toluene is recovered, the distillation under reduced pressure is stopped, 150 ml of ethanol is added, and 10 ml of ammonia water is added.0.5 g of activated carbon, stirred and dissolved for 30 minutes,Filter the activated carbon and slowly add 50 ml of pure water to the filtrate.Stir while stirring, product precipitation, filtration, hot water wash twice,Filtration and drying gave 21.4 g of 2-(4-chlorobenzyl)benzimidazole, the yield was 88.2%, and the product color was good. |
| 85% |
With boric acid; In 5,5-dimethyl-1,3-cyclohexadiene; for 16h;Reflux; |
General procedure: To a stirred solution of benzene-1,2-diamine 1 (1.85 mmol)in xylenes (10 mL) were added carboxylic acid 2 (2.77 mmol)and boric acid (0.185 mmol). The resulting solution wasrefluxed for 16 h. After cooling to room temperature, the reactionwas concentrated under reduced pressure and diluted withEtOAc (50 mL). The organic phase was washed with saturatedNaHCO3 solution (2 50 mL), dried over anhydrous Na2SO4and then concentrated under reduced pressure. The residuewas purified by silica gel flash column chromatography (elutingwith 10-15% Ethyl acetate in hexanes) to afford the title compounds3a-y and 5.6.2.5 2-(4-Chlorobenzyl)-1H-benzo[d]imidazole (3e) Yield 85%; Off white solid; mp 191-194 C; IR (KBr) 2850, 2751, 1512, 1451, 1410, 1241, 1181, 1025, 812, 749 cm-1; 1H NMR (400 MHz, DMSO-d6) delta 12.26 (br s, 1H), 7.52 (br s, 1H), 7.31-7.45 (m, 5H), 7.07-7.16 (m, 2H), 4.17 (s, 2H); 13C NMR (100 MHz, DMSO-d6) delta 153.2, 136.7, 134.5, 131.3, 130.8, 128.5, 121.8, 121.1, 118.3, 111.0, 34.1; HRMS calcd for C14H11ClN2 m/z 242.0625, found 242.0621. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping