Alternatived Products of [ 5096-21-9 ]
Product Details of [ 5096-21-9 ]
| CAS No. : | 5096-21-9 |
MDL No. : | MFCD00464247 |
| Formula : |
C11H15NO
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | - |
| M.W : |
177.24
|
Pubchem ID : | - |
| Synonyms : |
|
Safety of [ 5096-21-9 ]
| Signal Word: | |
Class: | |
| Precautionary Statements: | |
UN#: | |
| Hazard Statements: | |
Packing Group: | |
Application In Synthesis of [ 5096-21-9 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 5096-21-9 ]
- 1
-
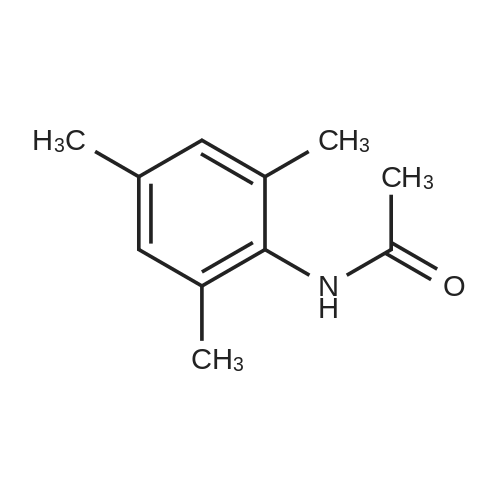 [ 5096-21-9 ]
[ 5096-21-9 ]

-
 [ 71182-65-5 ]
[ 71182-65-5 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With lithium aluminium tetrahydride |
|
- 2
-
 [ 108-24-7 ]
[ 108-24-7 ]

-
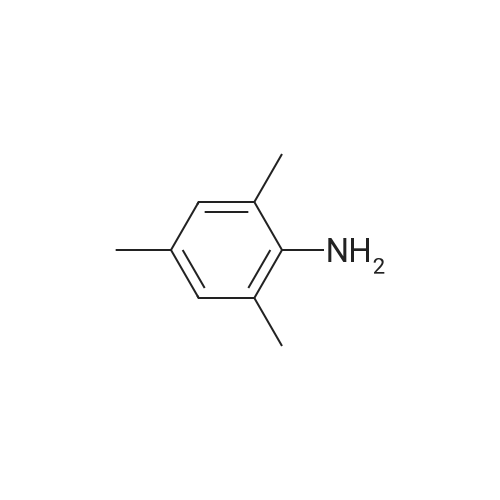 [ 88-05-1 ]
[ 88-05-1 ]

-
 [ 5096-21-9 ]
[ 5096-21-9 ]
| Yield | Reaction Conditions | Operation in experiment |
| 100% |
at 0 - 20℃; for 2.5h; Inert atmosphere; Schlenk technique; |
|
| 93% |
With sulfonic acid-functionalized hyper-cross-linked poly(2-naphthol) In neat (no solvent) at 25℃; for 0.166667h; Green chemistry; chemoselective reaction; |
2.3 General Procedure forAcylation Reaction
General procedure: To a 10mL reaction flask phenol/alcohol/thiol/amine/aldehyde(1mmol), acetic anhydride (1mmol) and p2NPh-OSO3H(10mg) as a catalyst was added and the resulting mixturestirred at 25°C. The progress of the reaction was checkedusing thin layer chromatography (TLC). After completion,ethyl acetate (EA) was added and the catalyst was separatedfrom the reaction mixture by straightforward filtration. Theseparated catalyst was cleaned with EA (10mL) and then driedin an oven for 2h and reused for further reaction. The reactionmixture was evaporated under reduced pressure to get theproducts which were characterized by 1H and 13C NMR spectroscopy.All of the obtained products are renowned withinthe literature. |
| 90% |
With N,N,N-trimethyl-2-(sulfooxy)ethanaminium chloride In neat (no solvent) at 20℃; for 0.216667h; Milling; Green chemistry; |
General procedure for the preparation of compounds (1-21).
General procedure: A mixture of amine (1 mmol), acetic anhydride (1 mmol) and 3.5 mol% SCIL was systematically mixed in a mortar followed by grinding at room temperature. The sticky mixture got solidified within 10-20 min. The mixture was powdered till the completion of reaction as indicated by TLC. The product was dissolved in ethyl acetate to recover the insoluble catalyst. The filtered catalyst was dried at 60oC under vaccum for 1 h after thoroughly washing with ethyl acetate. The recovered SCIL was recycled in the model reaction for six times to check its catalytic efficiency. The products were recrystallized from hot methanol. |
| 90% |
With pentaaminechlorocobalt(III) dichloride; phosphoric acid In neat (no solvent) at 75℃; for 0.166667h; Green chemistry; chemoselective reaction; |
|
|
With alkali |
|
|
With benzene |
|
|
With hydrogenchloride |
|

Reference:
[1]Van Dijk, Tom; Rong, Mark K.; Borger, Jaap E.; Nieger, Martin; Slootweg, J. Chris; Lammertsma, Koop
[Organometallics, 2016, vol. 35, # 5, p. 827 - 835]
[2]Kalla, Reddi Mohan Naidu; Reddy, Sirigireddy Sudharsan; Kim, Il
[Catalysis Letters, 2019, vol. 149, # 10, p. 2696 - 2705]
[3]Kalla, Reddi Mohan Naidu; Lim, Jaehwa; Bae, Jaeyeong; Kim, Il
[Tetrahedron Letters, 2017, vol. 58, # 16, p. 1595 - 1599]
[4]Sarief, Abdulla; Haque, SK Manirul; Feroze, Syed Mudabbir; Arifuddin, Mohammed
[Journal of the Chinese Chemical Society, 2018, vol. 65, # 9, p. 1104 - 1109]
[5]Ingold; Piggott
[Journal of the Chemical Society, 1924, vol. 125, p. 173]
[6]Dadswell; Kenner
[Journal of the Chemical Society, 1927, p. 1106]
[7]Zavlin; Efremov
[Russian Journal of General Chemistry, 1997, vol. 67, # 6, p. 872 - 877]
- 3
-
 [ 5096-21-9 ]
[ 5096-21-9 ]

-
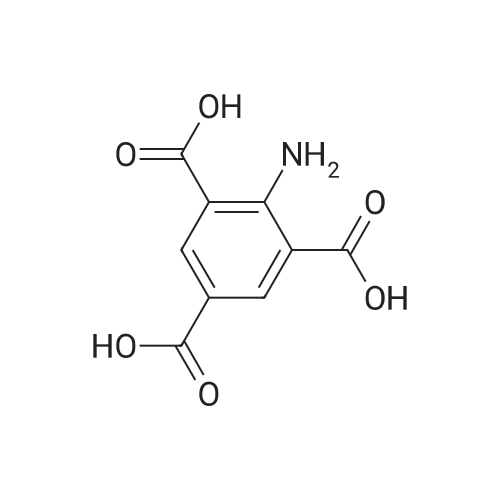 [ 489-96-3 ]
[ 489-96-3 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: aqueous KMnO4; aqueous MgSO4
2: aqueous H2SO4 |
|
| 3 g |
With potassium permanganate; sodium hydroxide In water at 25 - 85℃; for 75h; |
|
Reference:
[1]Quilico et al.
[Gazzetta Chimica Italiana, 1953, vol. 83, p. 179,181, 182]
[2]Rubin, Heather N.; Reynolds, Melissa M.
[Inorganic Chemistry, 2017, vol. 56, # 9, p. 5266 - 5274]
- 4
-
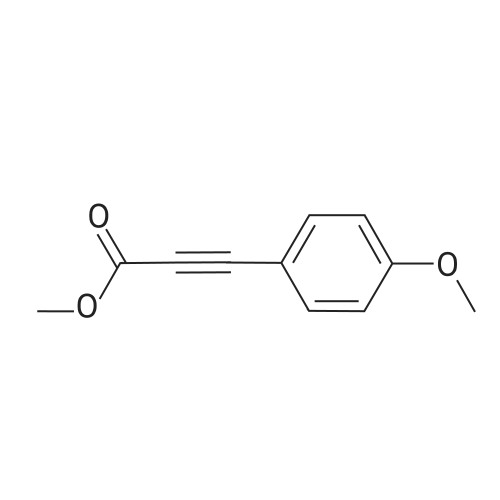 [ 7515-17-5 ]
[ 7515-17-5 ]

-
 [ 5096-21-9 ]
[ 5096-21-9 ]

-
 [ 1139719-79-1 ]
[ 1139719-79-1 ]
- 5
-
 [ 2050-43-3 ]
[ 2050-43-3 ]

-
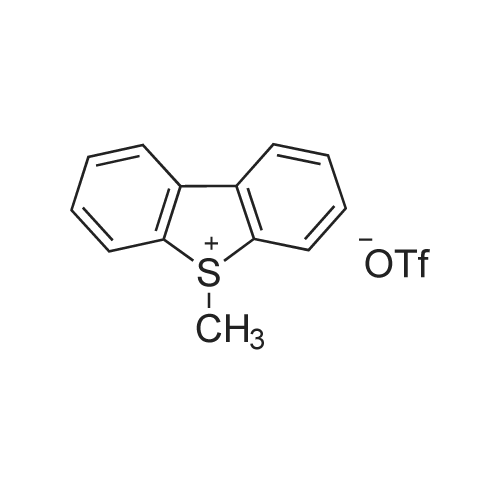 [ 112359-25-8 ]
[ 112359-25-8 ]

-
 [ 5096-21-9 ]
[ 5096-21-9 ]
| Yield | Reaction Conditions | Operation in experiment |
| 32% |
With palladium diacetate; copper(II) acetate monohydrate; trifluoroacetic acid In 1,2-dichloro-ethane at 50℃; for 16h; Sealed tube; |
|

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping










