| 85% |
With pyrrolidine; In tetrahydrofuran; at 20℃; |
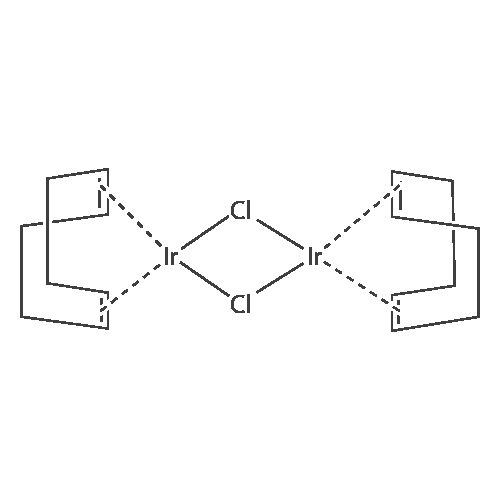
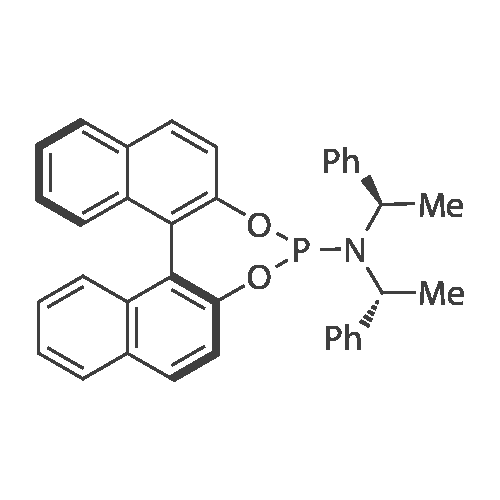
THF (5 mL) was added to a mixture of [[(COD) IRCL] (Ll) ] (1) [(100 MG,] 0. [110 MMOL) AND PHOSPHORAMIDITE LL] (123 mg, 0.230 mmol) at room temperature. After complete dissolution of the iridium complex, pyrrolidine (391 mg, 460 [J. L,] 5.50 mmol) was added by syringe, and the solution was strirred overnight. The color of the solution changed from orange to yellow, and a precipitate of the pyrrolidine hydrochloride was observed. The solvent was evaporated under vacuum, and the yellow residue was suspended in a mixture of 2 mL diethyl ether and 2 mL benzene. This suspension was filtered through a 0.45 [PM] nylon syringe filter. The solvent was evaporated under vacuum, and the yellow product was dissolved in 0.5 mL CH2C12 and precipitated by layering with pentane at-30C to yield a fine yellow powder, which was dried under high vacuum overnight. Yield: 85 % (129 [MG). LH NMR] (400.13 MHz, [CD2CK,] 248 K) : [8-0.] 66 (dt, J= 11.8, 6.0 Hz, [1H,] [IRCH2),] 0.32 (br s, 3H, [CH3),] 0.92 (d, [J=] 6.8 Hz, 3H, [CH3),] 1. [37] (d, [J=] 6.5 Hz, 3H, CHCH3), 1.67 [(M,] [1H,] COD), 1.73 (dt, [J=] 18.6, 11.3 [HZ, 1H,] IrCH2), 1.93 [(M,] 2H, COD), 2.32 [(M,] 2H, COD), 2.45 [(M,] 2H, COD), 2.71 [(M.] 1H, COD), 2.91 [(M,] 2H, COD), 3. 28 (dd, J= 6.9 Hz, J= 7.1 Hz, [1H,] CHCH2Ir), 3.75 [(M,] 1H, CHCH3), 3.81 [(M,] 1H, COD), 4.54 [(M,] 1H, CHCH3), 4.69 [(M,] 1H, COD), 5.11 [(M,] 1H, CHCH3), 5.85 (d, J= 7.2 Hz, 1H, ArH), 6.50 (d, J= 9.5 Hz, 1H, ArH), 6.55 (t, J= 12.6 Hz, 2H, ArH), 6.92 [(M,] 4H, ArH), 6.92 (d, J= 7.0 Hz, 1H, ArH), 7.08 [(M,] 2H, ArH), 7.13 [(M,] 3H, ArH), 7.17-7. 26 [(M,] 13H, ArH), 7.32 [(M,] 4H, [ARH),] 7.40 [(M,] 2H, ArH), 7.49 (t, J= 8.7 Hz, 1H, [ARH),] 7.64 (d, J= 8.6 Hz, 1H, ArH), 7.73 (d, J= 8.7 Hz, 2H, ArH), 7.83 (d, J='8. 5 Hz, 1H, ArH), 7.87 (d, J= 8.7 Hz, 1H, ArH), 7.92-8. 06 [(M,] 5H, ArH), 8.13 (d, J= 8.6 Hz, 1H, ArH); 31p NMR (161.9 MHz, [CD2C12) 6] 152.6 (d, J= 46.3 Hz, [1P),] 127.8 (d, J= 46.2 Hz, [1P)] main diastereomer (94 %), 149.4 (d, [J=] 77.8 Hz, [1P),] 146.0 (d, J= 78. 0 Hz, [1P)] minor diastereomer (6 %); Aliphatic region of the [13C] NMR spectrum (125.7 MHz, [CD2C12,] 248K) 8 15.2 (dd, [J=] 4.9 Hz, [J=] 5.8 Hz, IrCH2), 20.1 (s, [CH3),] 21.9 (s, [CH3),] 24.1 (s, [CH3),] 23.7 (br s, CH2-COD), 29.7 (br s, CH2-COD), 34.3 (br s, CH2-COD), 44.0 (s, [CH2-COD),] 45.7 [(M,] CH-COD), 46.2 [(M,] CH-COD), 53.9 (s, CH), 54.7 (d, J= 24.7 Hz, CH), 59.0 (br s, CH), 68.3 (d, [J=] 43.9 Hz, [CHCH2),] 69.8 (s, CH- COD), 82.6 (s, CH-COD); MS [(FAB+)] (%): [M/Z =] 1378.1 [M+] (8), 838.2 [M+- [C36H3OP02N]] (100), 730.2 [[M+-L1-CSHL2]] (69); Anal. Calc. for [CSOH7IH-N204P2] : C, 69.70 ; H, 5.19 ; N, 2.03 ; P, 4.49 ; [CL,] 0.00. Found: C, 69.55 ; H, 5.35 ; N, 2.09 ; P, 4.36, Cl <0.02. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping