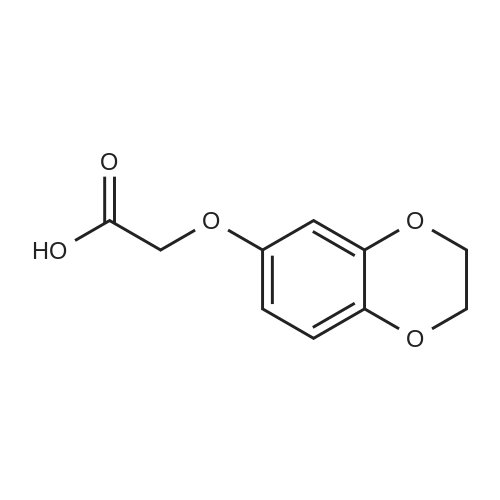
Alternatived Products of [ 4385-48-2 ]
Product Details of [ 4385-48-2 ]
| CAS No. : | 4385-48-2 |
MDL No. : | MFCD00187272 |
| Formula : |
C8H6O3
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | ULEKGOXADQVOIF-UHFFFAOYSA-N |
| M.W : |
150.13
|
Pubchem ID : | 4685450 |
| Synonyms : |
|
Application In Synthesis of [ 4385-48-2 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 4385-48-2 ]
- 1
-
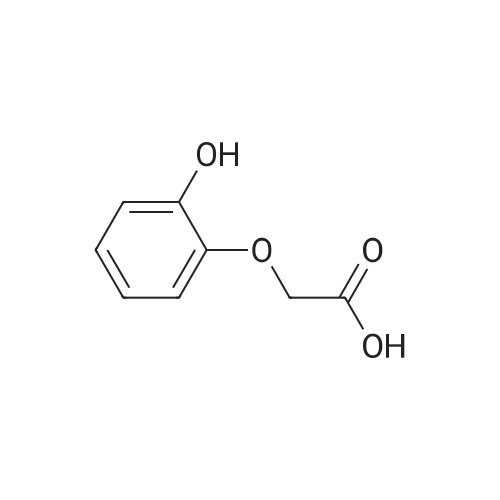 [ 6324-11-4 ]
[ 6324-11-4 ]

-
 [ 4385-48-2 ]
[ 4385-48-2 ]
| Yield | Reaction Conditions | Operation in experiment |
|
bei der Destillation; |
|
|
bei der Destillation; |
|
Reference:
[1]Moureu
[Annales de Chimie (Cachan, France), 1899, vol. <7> 18, p. 130]
Current Patent Assignee: Tobias - DE89593, 1800, C
[Fortschr. Teerfarbenfabr. Verw. Industriezweige, vol. 4, p. 1109][Fortschr. Teerfarbenfabr. Verw. Industriezweige, vol. 4, p. 1109]
Moureu
[Comptes Rendus Hebdomadaires des Seances de l'Academie des Sciences, 1898, vol. 127, p. 227][Bulletin de la Societe Chimique de France, 1899, vol. <3> 21, p. 104]
Ludewig
[Journal fur praktische Chemie (Leipzig 1954), 1900, vol. <2> 61, p. 360]
Carter; Lawrence
[Journal of the Chemical Society, 1900, vol. 77, p. 1224]
[2]Current Patent Assignee: Majert - DE87336, 1800, C
[Fortschr. Teerfarbenfabr. Verw. Industriezweige, vol. 4, p. 1107][Fortschr. Teerfarbenfabr. Verw. Industriezweige, vol. 4, p. 1107]
- 2
-
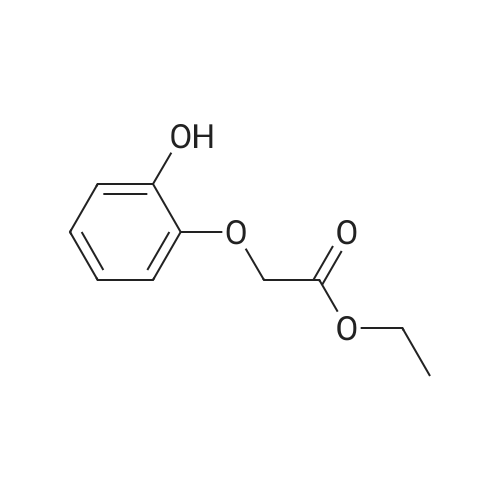 [ 99186-63-7 ]
[ 99186-63-7 ]

-
 [ 4385-48-2 ]
[ 4385-48-2 ]
- 3
-
 [ 120-80-9 ]
[ 120-80-9 ]

-
 [ 79-04-9 ]
[ 79-04-9 ]

-
 [ 4385-48-2 ]
[ 4385-48-2 ]
| Yield | Reaction Conditions | Operation in experiment |
| 70% |
With TEA In dichloromethane for 4h; Heating; |
|
|
With calcium carbonate |
|
|
With triethylamine In toluene for 3h; Heating; |
|
Reference:
[1]Gandolfi, Carmelo A.; Domenico, Roberto Di; Spinelli, Silvano; Gallico, Licia; Fiocchi, Luigi; et al.
[Journal of Medicinal Chemistry, 1995, vol. 38, # 3, p. 508 - 525]
[2]Current Patent Assignee: Chem. Fabr. v. Heyden; zitiert von Friedlaender
[Fortschr. Teerfarbenfabr. Verw. Industriezweige, vol. 4, p. 1109]
[3]Sampson, Peter; Crook, Michelle; Piorko, Adam
[Journal of Heterocyclic Chemistry, 1994, vol. 31, # 4, p. 1011 - 1016]
- 4
-
 [ 4385-48-2 ]
[ 4385-48-2 ]

-
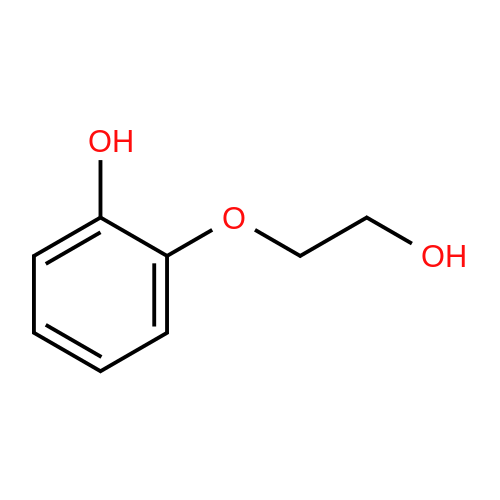 [ 4792-78-3 ]
[ 4792-78-3 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With copper oxide-chromium oxide at 250℃; Hydrogenolyse; |
|
- 5
-
 [ 4385-48-2 ]
[ 4385-48-2 ]

-
 [ 75724-80-0 ]
[ 75724-80-0 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With nitric acid; acetic anhydride |
|
- 6
-
 [ 4385-48-2 ]
[ 4385-48-2 ]

-
trans 2-(2-hydroxyethoxy)cyclohexanol
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
With nickel at 200℃; Hydrogenation; |
|
|
Multi-step reaction with 2 steps
1: copper oxide-chromium oxide / 250 °C / 73550.8 - 147102 Torr / Hydrogenolyse
2: Raney nickel / 200 °C / 73550.8 - 147102 Torr / Hydrogenation |
|
- 7
-
 [ 4385-48-2 ]
[ 4385-48-2 ]

-
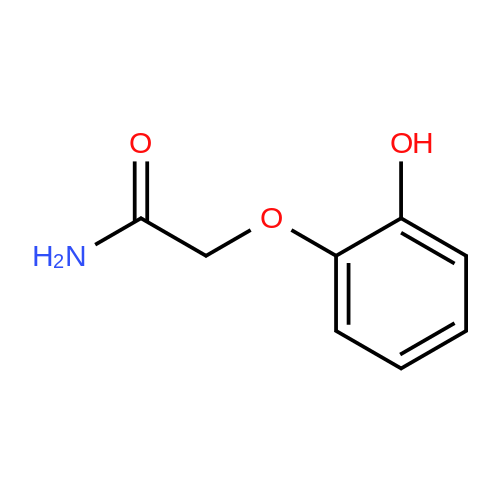 [ 34919-87-4 ]
[ 34919-87-4 ]
| Yield | Reaction Conditions | Operation in experiment |
| 63% |
With ammonia In water for 2h; |
|
|
With ammonium hydroxide |
|
Reference:
[1]Anisimova; Turchin; Golovanova; Vinogradova; Yuzhakov; Mashkovskii; Pleshkova; Sheinker
[Pharmaceutical Chemistry Journal, 1997, vol. 31, # 5, p. 224 - 228]
[2]Current Patent Assignee: AKZO NOBEL NV - DE2126169, 1971, A1
[Chem.Abstr., 1972, vol. 76, # 72289][Chem.Abstr., 1972, vol. 76, # 72289]
- 8
-
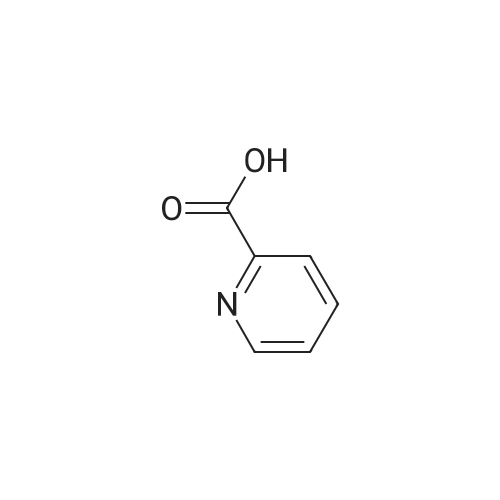 [ 98-98-6 ]
[ 98-98-6 ]

-
 [ 4385-48-2 ]
[ 4385-48-2 ]

-
 [ 103681-70-5 ]
[ 103681-70-5 ]
| Yield | Reaction Conditions | Operation in experiment |
| 51 % Turnov. |
In various solvent(s) for 3h; Heating; |
|
| 51 % Turnov. |
With 4-methylisopropylbenzene Heating; |
|
Reference:
[1]Dzvinchuk, I. B.; Lozinskii, M. O.
[Chemistry of Heterocyclic Compounds, 1985, vol. 21, # 11, p. 1295][Khimiya Geterotsiklicheskikh Soedinenii, 1985, vol. 21, # 11, p. 1570]
[2]Dzvinchuk, I. B.; Sereda, S. V.; Lozinskii, M. O.; Struchkov, Yu. T.
[Journal of Organic Chemistry USSR (English Translation), 1991, vol. 27, # 5.2, p. 914 - 920][Zhurnal Organicheskoi Khimii, 1991, vol. 27, # 5, p. 1058 - 1065]
- 9
-
 [ 67-56-1 ]
[ 67-56-1 ]

-
 [ 6324-11-4 ]
[ 6324-11-4 ]

-
 [ 4385-48-2 ]
[ 4385-48-2 ]
| Yield | Reaction Conditions | Operation in experiment |
| 18% |
With toluene-4-sulfonic acid In benzene for 3h; Heating; |
|
- 10
-
 [ 4385-48-2 ]
[ 4385-48-2 ]

-
 [ 34920-08-6 ]
[ 34920-08-6 ]

-
7-bromo-1,4-benzodioxin-2(3H)-one
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 1: 4%
2: 13% |
With N-Bromosuccinimide In tetrahydrofuran for 0.5h; |
|
| 11% |
With N-Bromosuccinimide; Perbenzoic acid In tetrahydrofuran for 3h; Irradiation; |
|
Reference:
[1]Sampson, Peter; Crook, Michelle; Piorko, Adam
[Journal of Heterocyclic Chemistry, 1994, vol. 31, # 4, p. 1011 - 1016]
[2]Sampson, Peter; Crook, Michelle; Piorko, Adam
[Journal of Heterocyclic Chemistry, 1994, vol. 31, # 4, p. 1011 - 1016]
- 11
-
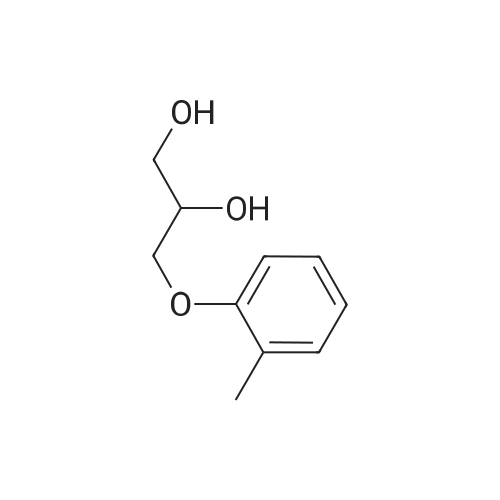 [ 59-47-2 ]
[ 59-47-2 ]

-
 [ 4385-48-2 ]
[ 4385-48-2 ]

-
 [ 17753-05-8 ]
[ 17753-05-8 ]
- 12
-
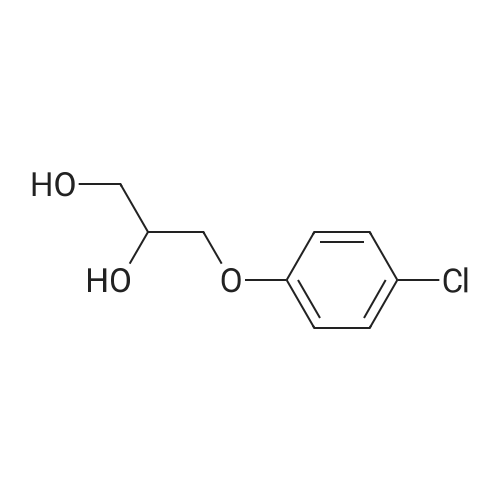 [ 104-29-0 ]
[ 104-29-0 ]

-
 [ 4385-48-2 ]
[ 4385-48-2 ]

-
 [ 39719-58-9 ]
[ 39719-58-9 ]
- 13
-
 [ 4385-48-2 ]
[ 4385-48-2 ]

-
 [ 101421-67-4 ]
[ 101421-67-4 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With nitric acid; acetic anhydride |
|
- 14
-
 [ 4385-48-2 ]
[ 4385-48-2 ]

-
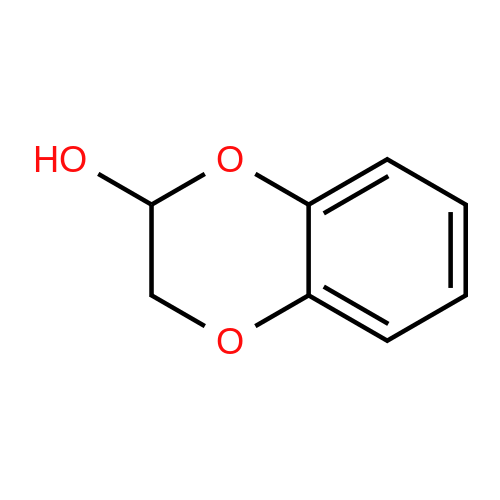 [ 5770-59-2 ]
[ 5770-59-2 ]
| Yield | Reaction Conditions | Operation in experiment |
| 95% |
With diisobutylaluminium hydride In toluene at -70℃; for 1h; |
|
- 15
-
 [ 4385-48-2 ]
[ 4385-48-2 ]

-
 [ 107-15-3 ]
[ 107-15-3 ]

-
 [ 53259-03-3 ]
[ 53259-03-3 ]
| Yield | Reaction Conditions | Operation in experiment |
|
In N,N-dimethyl-formamide |
|
- 16
-
 [ 4385-48-2 ]
[ 4385-48-2 ]

-
 [ 4792-78-3 ]
[ 4792-78-3 ]
| Yield | Reaction Conditions | Operation in experiment |
|
at 250℃; Hydrogenation; |
|
- 17
-
 [ 4385-48-2 ]
[ 4385-48-2 ]

-
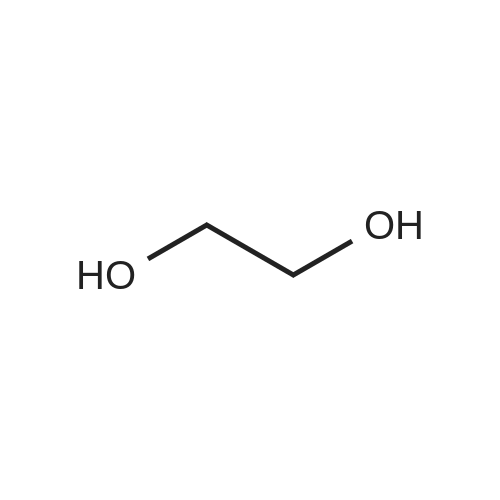 [ 107-21-1 ]
[ 107-21-1 ]

-
 [ 108-93-0 ]
[ 108-93-0 ]
| Yield | Reaction Conditions | Operation in experiment |
|
at 200℃; Hydrogenation; |
|
- 18
-
 [ 4385-48-2 ]
[ 4385-48-2 ]

-
 [ 137-07-5 ]
[ 137-07-5 ]

-
2-(o-hydroxyphenoxymethyl)benzothiazole
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 94% |
In o-xylene Heating; |
|
- 19
-
 [ 4385-48-2 ]
[ 4385-48-2 ]

-
 [ 183283-10-5 ]
[ 183283-10-5 ]

-
7-[2-(4,5-dihydro-1H-imidazol-2-yl)-1,2-dihydrophthalazin-1-yl]-1,4-benzodioxin-2(3H)-one
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 42% |
With hydrogenchloride; methanol at 20℃; for 72h; |
|
- 20
-
 [ 856190-95-9 ]
[ 856190-95-9 ]

-
 [ 4385-48-2 ]
[ 4385-48-2 ]

-
 [ 856190-96-0 ]
[ 856190-96-0 ]
| Yield | Reaction Conditions | Operation in experiment |
| 76% |
In dichloromethane at 0 - 20℃; for 3h; |
|
Reference:
[1]Brittain, Dominic E. A.; Griffiths-Jones, Charlotte M.; Linder, Michael R.; Smith, Martin D.; McCusker, Catherine; Barlow, Jaqueline S.; Akiyama, Ryo; Yasuda, Kosuke; Ley, Steven V.
[Angewandte Chemie - International Edition, 2005, vol. 44, # 18, p. 2732 - 2737]
- 21
-
 [ 4385-48-2 ]
[ 4385-48-2 ]

-
2-(o-hydroxyphenoxymethyl)-3-methylbenzothiazolium methyl sulfate
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: 94 percent / o-xylene / Heating
2: 91 percent / propan-2-ol / 1 h / Heating |
|
- 22
-
 [ 4385-48-2 ]
[ 4385-48-2 ]

-
[2-(2,3-epoxypropoxy)phenoxy]acetamide
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: 63 percent / conc. aq. amonia solution / H2O / 2 h
2: NaOH / dioxane; H2O / 3 h / 70 - 80 °C |
|
- 23
-
 [ 4385-48-2 ]
[ 4385-48-2 ]

-
 [ 34920-03-1 ]
[ 34920-03-1 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 3 steps
1: 63 percent / conc. aq. amonia solution / H2O / 2 h
2: NaOH / dioxane; H2O / 3 h / 70 - 80 °C
3: 44 percent / methanol / 3 h / Heating |
|
- 24
-
 [ 4385-48-2 ]
[ 4385-48-2 ]

-
[2-(3-tert-butylamino-2-hydroxypropoxy)phenoxy]acetic acid
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 4 steps
1: 63 percent / conc. aq. amonia solution / H2O / 2 h
2: NaOH / dioxane; H2O / 3 h / 70 - 80 °C
3: 44 percent / methanol / 3 h / Heating
4: NaOH / H2O / 5 h / 90 - 95 °C / Alkaline hydrolysis |
|
- 25
-
 [ 120-80-9 ]
[ 120-80-9 ]

-
 [ 4385-48-2 ]
[ 4385-48-2 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 3 steps
1: EtONa / ethanol / 16 h / Heating
2: 21 percent / conc. HCl / 1 h / Heating
3: 18 percent / PTSA / benzene / 3 h / Heating |
|
- 26
-
 [ 4385-48-2 ]
[ 4385-48-2 ]

-
(2-acetoxy-phenoxy)-acetic acid anilide
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: diethyl ether |
|
- 27
-
 [ 4385-48-2 ]
[ 4385-48-2 ]

-
(2-benzoyloxy-phenoxy)-acetic acid anilide
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: diethyl ether
2: natrium carbonate |
|
- 28
-
 [ 4385-48-2 ]
[ 4385-48-2 ]

-
 [ 34919-96-5 ]
[ 34919-96-5 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: water
2: (i) aq. NaOH, (ii) aq. NH3 |
|
- 29
-
 [ 4385-48-2 ]
[ 4385-48-2 ]

-
 [ 60787-30-6 ]
[ 60787-30-6 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: water
2: (i) aq. NaOH, (ii) /BRN= 605259/ |
|
|
Multi-step reaction with 3 steps
1: water
2: (i) aq. NaOH, (ii) aq. NH3
3: hydrogen / palladium on activated charcoal / ethanol / 50 °C / 36775.4 Torr |
|
Reference:
[1]Current Patent Assignee: AKZO NOBEL NV - DE2126169, 1971, A1
[Chem.Abstr., 1972, vol. 76, # 72289][Chem.Abstr., 1972, vol. 76, # 72289]
[2]Current Patent Assignee: AKZO NOBEL NV - DE2126169, 1971, A1
[Chem.Abstr., 1972, vol. 76, # 72289][Chem.Abstr., 1972, vol. 76, # 72289]
- 30
-
 [ 4385-48-2 ]
[ 4385-48-2 ]

-
 [ 34920-13-3 ]
[ 34920-13-3 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 3 steps
1: water
2: (i) aq. NaOH, (ii) /BRN= 635703/
3: hydrogen / palladium on activated charcoal / ethanol |
|
- 31
-
 [ 4385-48-2 ]
[ 4385-48-2 ]

-
 [ 34920-14-4 ]
[ 34920-14-4 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 3 steps
2: (i) aq. NaOH, (ii) /BRN= 605259/
3: hydrogen / palladium on activated charcoal / ethanol |
|
- 32
-
 [ 4385-48-2 ]
[ 4385-48-2 ]

-
 [ 103095-46-1 ]
[ 103095-46-1 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: acetic acid anhydride; nitric acid
2: ethanolic H2SO4 |
|
|
Multi-step reaction with 3 steps
1: acetic acid anhydride; nitric acid
2: H2O
3: ethanolic H2SO4 |
|
- 33
-
 [ 4385-48-2 ]
[ 4385-48-2 ]

-
 [ 98594-63-9 ]
[ 98594-63-9 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 4 steps
1: acetic acid anhydride; nitric acid
2: ethanolic H2SO4
3: palladium/charcoal / Hydrogenation
4: ethanol; N2H4+H2O |
|
|
Multi-step reaction with 5 steps
1: acetic acid anhydride; nitric acid
2: H2O
3: ethanolic H2SO4
4: palladium/charcoal / Hydrogenation
5: ethanol; N2H4+H2O |
|
- 34
-
 [ 4385-48-2 ]
[ 4385-48-2 ]

-
 [ 99076-31-0 ]
[ 99076-31-0 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 3 steps
1: acetic acid anhydride; nitric acid
2: ethanolic H2SO4
3: palladium/charcoal / Hydrogenation |
|
|
Multi-step reaction with 4 steps
1: acetic acid anhydride; nitric acid
2: H2O
3: ethanolic H2SO4
4: palladium/charcoal / Hydrogenation |
|
- 35
-
 [ 4385-48-2 ]
[ 4385-48-2 ]

-
 [ 100959-20-4 ]
[ 100959-20-4 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: HNO3, Ac2O
2: HNO3 |
|
|
Multi-step reaction with 3 steps
1: HNO3, Ac2O
2: HNO3 / acetic acid
3: HNO3 / acetic acid |
|
- 36
-
 [ 4385-48-2 ]
[ 4385-48-2 ]

-
 [ 100949-08-4 ]
[ 100949-08-4 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: HNO3, Ac2O
2: HNO3 / acetic acid |
|
- 37
-
 [ 4385-48-2 ]
[ 4385-48-2 ]

-
 [ 53259-05-5 ]
[ 53259-05-5 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: dimethylformamide
2: aq. KOH / ethanol |
|
- 38
-
 [ 4385-48-2 ]
[ 4385-48-2 ]

-
 [ 53259-06-6 ]
[ 53259-06-6 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: dimethylformamide
2: aq. NaOH |
|
- 39
-
 [ 4385-48-2 ]
[ 4385-48-2 ]

-
 [ 53259-04-4 ]
[ 53259-04-4 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: dimethylformamide
2: aq. KOH / ethanol |
|
- 40
-
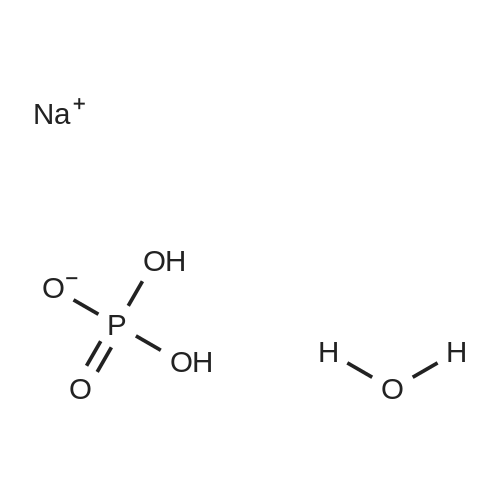 [ 10049-21-5 ]
[ 10049-21-5 ]

-
2N-isopropanol
[ No CAS ]

-
Na 2 SO4
[ No CAS ]

-
 [ 4385-48-2 ]
[ 4385-48-2 ]

-
 [ 5770-59-2 ]
[ 5770-59-2 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With diisobutylaluminium hydride In toluene |
6 EXAMPLE 6
A molar solution of DIBAH in toluene (73 ml) is added dropwise to a stirred solution of 1,4-benzodioxan-2-one (8.9 g) in dry toluene (100 ml), cooled at -70° C. during 40 minutes. Stirring at this temperature is continued for 15 minutes, then the excess reagent is destroyed by adding 2N-isopropanol in toluene (75 ml), under stirring, at -70°÷-60° C. The mixture is warmed at room temperature and treated with 30% NaH2 PO4 aqueous solution (6 ml) and 25 g of anhydrous Na 2 SO4, for 4 hours, under stirring. The inorganic material is filtered out and the elude is evaporated to dryness to give 8.2 g of 2-hydroxy-1,4-benzodioxan. |
- 41
-
 [ 4385-48-2 ]
[ 4385-48-2 ]

-
 [ 58027-12-6 ]
[ 58027-12-6 ]

-
 [ 34919-78-3 ]
[ 34919-78-3 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With methylamine |
8 EXAMPLE 8
The 1-(o-N-methylcarbamoylmethoxyphenoxy)-2,3-epoxypropane used as starting material may be obtained as follows: 3 G. of 1,4-benzodioxan-2-one are added portionwise to a stirred, ice-cold, 27% w/v aqueous solution of methylamine, the temperature of the mixture being kept below 10°C., and after addition is complete the mixture is stirred at room temperature for a further 2 hours. The solution is evaporated to dryness under reduced pressure and the residue is crystallized from water. There is thus obtained o-hydroxyphenoxy-N-methylacetamide, m.p. 149°-150°C. |
|
With methylamine |
2 EXAMPLE 2
The 1-(o-N-methylcarbamoylmethoxyphenoxy)-2,3-epoxypropane used as starting material may be obtained as follows: 3 G. of 1,4-benzodioxan-2one are added portion-wise to a stirred, ice-cold, 27% w/v aqueous solution of methylamine, the temperature of the mixture being kept below 10° C., and after addition is complete the mixture is stirred at room temperature for a further 2 hours. The solution is evaporated to dryness under reduced pressure and the residue is crystallized from water. There is thus obtained o-hydroxyphenoxy-N-methylacetamide, m.p. 149°-150° C. |
- 42
-
 [ 4385-48-2 ]
[ 4385-48-2 ]

-
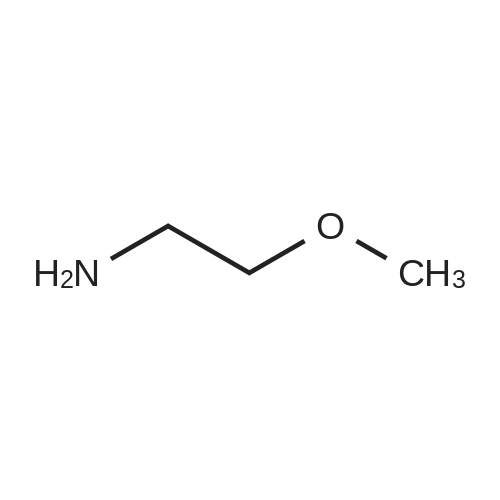 [ 109-85-3 ]
[ 109-85-3 ]

-
1-o-(N-β-methoxyethylcarbamoylmethoxy)phenoxy-3-isopropylamino-2-propanol
[ No CAS ]

-
N-β-methoxyethyl-o-hydroxyphenoxyacetamide
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
|
17 EXAMPLE 17
EXAMPLE 17 The process described in Example 16 is repeated except that N-β-methoxyethyl-o-hydroxyphenoxyacetamide (m.p. 96°-97°C. after crystallisation from water; prepared from 1,4-benzodioxan-2-one and 2-methoxyethylamine by a similar process to that described in the second part of Example 16) is used as starting material in place of N-β-hydroxyethyl-o-hydroxyphenoxyacetamide. There is thus obtained 1-o-(N-β-methoxyethylcarbamoylmethoxy)phenoxy-3-isopropylamino-2-propanol, m.p. 78°-80°C. after crystallisation from a mixture of ethyl acetate and petroleum ether (b.p. 60°-80°C.). |
- 43
-
o-(2,3-epoxypropoxy)phenyl-N-γ-hydroxypropoxyacetamide
[ No CAS ]

-
 [ 4385-48-2 ]
[ 4385-48-2 ]

-
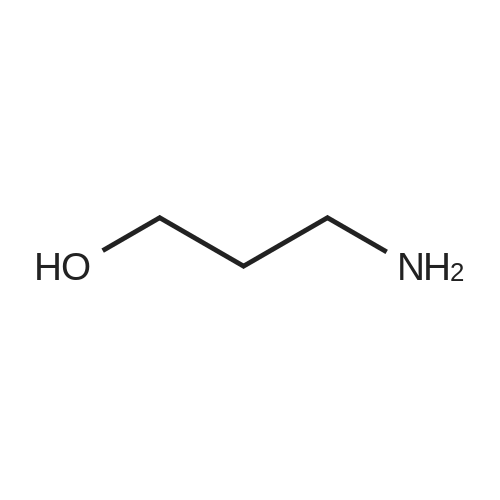 [ 156-87-6 ]
[ 156-87-6 ]

-
 [ 1153062-67-9 ]
[ 1153062-67-9 ]
| Yield | Reaction Conditions | Operation in experiment |
|
In ethanol |
21 EXAMPLE 21
The o-(2,3-epoxypropoxy)phenyl-N-γ-hydroxypropoxyacetamide used as starting material may be obtained as follows: A solution of 3-aminopropanol (9 ml.) and 1,4-benzodioxan-2-one (20 g.) in ethanol (150 ml.) is kept at laboratory temperature for 18 hours and then evaporated to dryness under reduced pressure. The residue is crystallized from water and there is thus obtained N-γ-hydroxypropyl-o-hydroxyphenoxyacetamide, m.p. 96°-98°C. |
- 44
-
 [ 141-43-5 ]
[ 141-43-5 ]

-
 [ 4385-48-2 ]
[ 4385-48-2 ]

-
N-β-hydroxyethyl-o-hydroxyphenoxyacetamide
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
In ethanol |
18 EXAMPLE 18
EXAMPLE 18 2-Aminoethanol (80 ml.) is added to a stirred solution of 1,4-benzodioxan-2-one (100 g.) in ethanol (1,500 ml.) at such a rate that the temperature of the mixture does not rise above 30°C. The mixture is then kept for a further 2 hours at laboratory temperature and then for 12 hours at 0°C. The mixture is filtered and the solid residue is crystallized from isopropanol. There is thus obtained N-β-hydroxyethyl-o-hydroxyphenoxyacetamide, m.p. 124°-126°C. |
- 45
-
 [ 4385-48-2 ]
[ 4385-48-2 ]

-
 [ 1360546-24-2 ]
[ 1360546-24-2 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 4 steps
1: aluminum (III) chloride; triethylamine / 1,2-dichloro-ethane / 12.25 h / 20 °C / Inert atmosphere
2: pyridine / dichloromethane / 0 °C / Inert atmosphere
3: 1,3-bis-(diphenylphosphino)propane; palladium diacetate; triethylamine / N,N-dimethyl-formamide / 40 h / 100 °C / Inert atmosphere
4: Lawessons reagent / tetrahydrofuran / 70 h / 20 °C |
|
- 46
-
 [ 4385-48-2 ]
[ 4385-48-2 ]

-
C27H27NO3S
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 5 steps
1: aluminum (III) chloride; triethylamine / 1,2-dichloro-ethane / 12.25 h / 20 °C / Inert atmosphere
2: pyridine / dichloromethane / 0 °C / Inert atmosphere
3: 1,3-bis-(diphenylphosphino)propane; palladium diacetate; triethylamine / N,N-dimethyl-formamide / 40 h / 100 °C / Inert atmosphere
4: Lawessons reagent / tetrahydrofuran / 70 h / 20 °C
5: [Cu(acetonitrile)4]SbF6; lithium 4-methoxyphenoxide; 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl / tetrahydrofuran; N,N-dimethyl-formamide / -40 °C / Inert atmosphere |
|
- 47
-
 [ 4385-48-2 ]
[ 4385-48-2 ]

-
C27H27NO3S
[ No CAS ]

-
 [ 1360546-34-4 ]
[ 1360546-34-4 ]

-
C27H27NO3S
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 5 steps
1: aluminum (III) chloride; triethylamine / 1,2-dichloro-ethane / 12.25 h / 20 °C / Inert atmosphere
2: pyridine / dichloromethane / 0 °C / Inert atmosphere
3: 1,3-bis-(diphenylphosphino)propane; palladium diacetate; triethylamine / N,N-dimethyl-formamide / 40 h / 100 °C / Inert atmosphere
4: Lawessons reagent / tetrahydrofuran / 70 h / 20 °C
5: [Cu(acetonitrile)4]SbF6; lithium 4-methoxyphenoxide; (S)-2,2',6,6'-tetramethoxy-4,4'-bis(di(3,5-xylyl)phosphino)-3,3'-bipyridine / tetrahydrofuran; N,N-dimethyl-formamide / 20 h / -40 °C / Inert atmosphere |
|
- 48
-
 [ 4385-48-2 ]
[ 4385-48-2 ]

-
C23H20F3NO5S
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: aluminum (III) chloride; triethylamine / 1,2-dichloro-ethane / 12.25 h / 20 °C / Inert atmosphere
2: pyridine / dichloromethane / 0 °C / Inert atmosphere |
|
- 49
-
 [ 4385-48-2 ]
[ 4385-48-2 ]

-
C27H27NO4
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 3 steps
1: aluminum (III) chloride; triethylamine / 1,2-dichloro-ethane / 12.25 h / 20 °C / Inert atmosphere
2: pyridine / dichloromethane / 0 °C / Inert atmosphere
3: 1,3-bis-(diphenylphosphino)propane; palladium diacetate; triethylamine / N,N-dimethyl-formamide / 40 h / 100 °C / Inert atmosphere |
|
- 50
-
 [ 4385-48-2 ]
[ 4385-48-2 ]

-
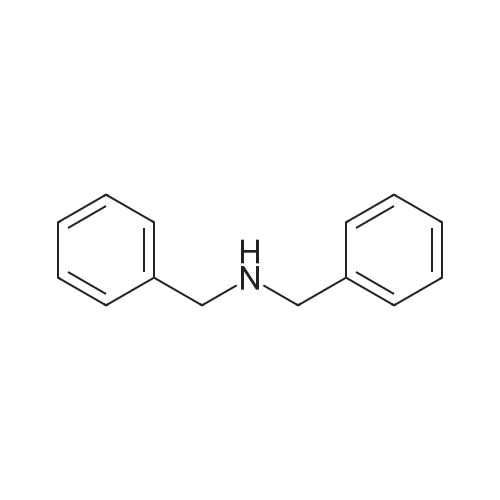 [ 103-49-1 ]
[ 103-49-1 ]

-
C22H21NO3
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
With aluminum (III) chloride; triethylamine In 1,2-dichloro-ethane at 20℃; for 12.25h; Inert atmosphere; |
|

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping