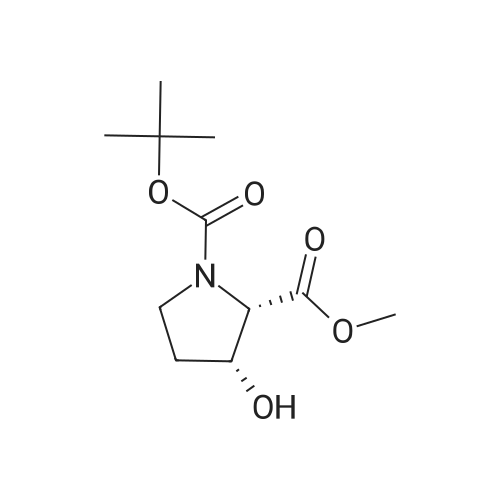
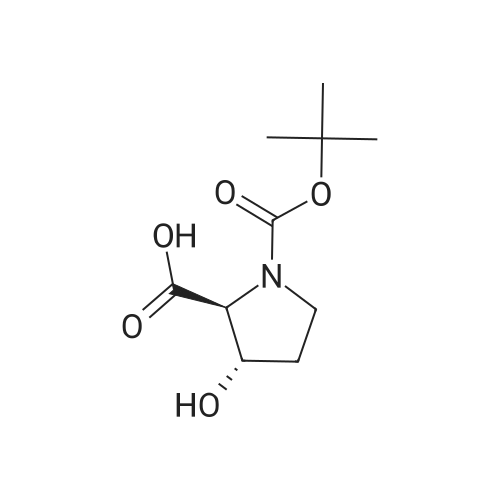
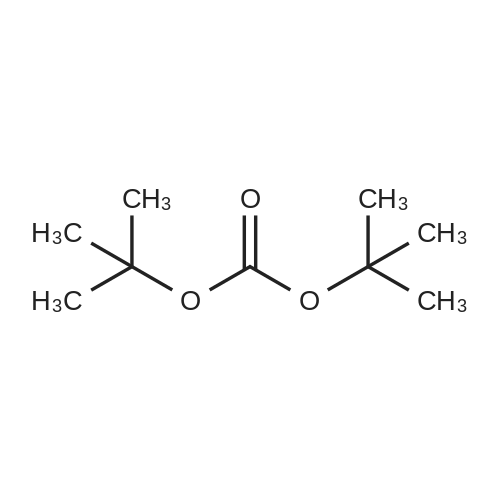
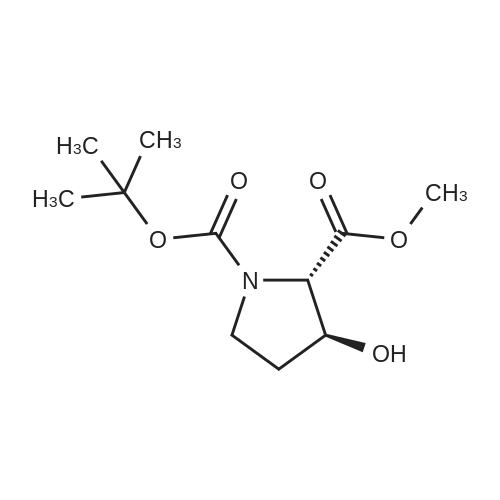
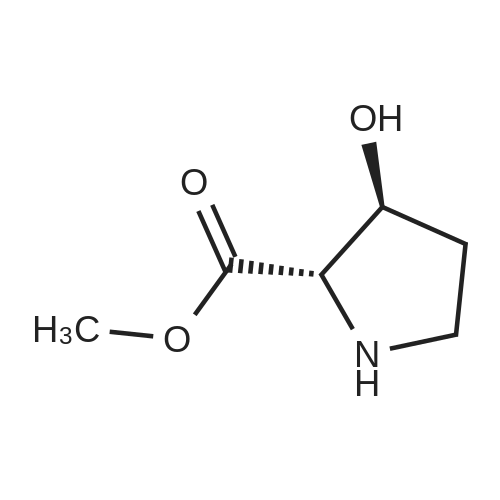
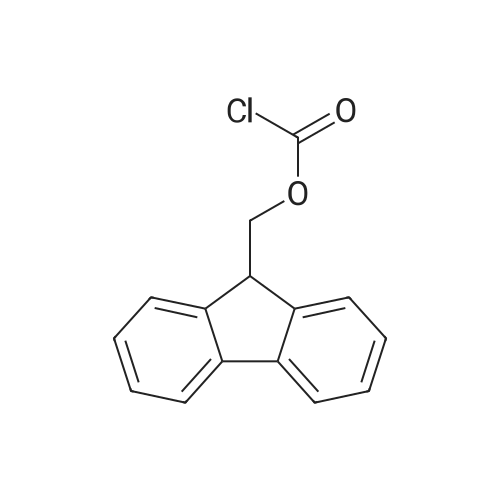
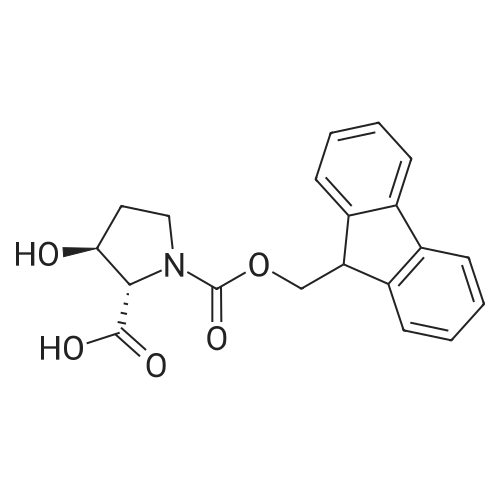
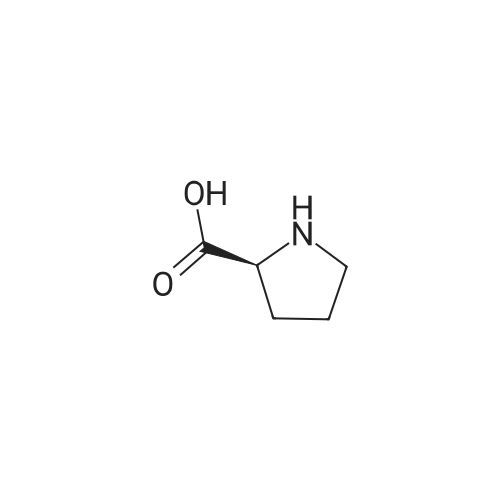
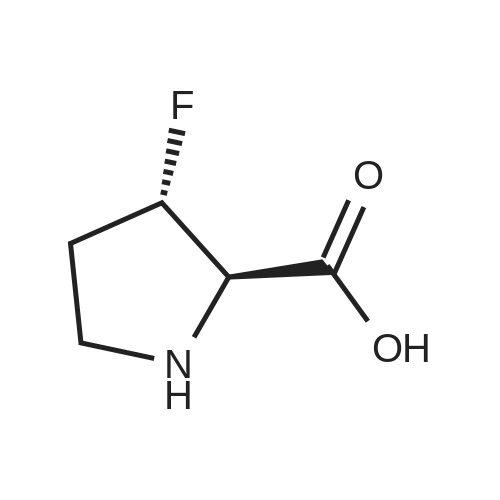
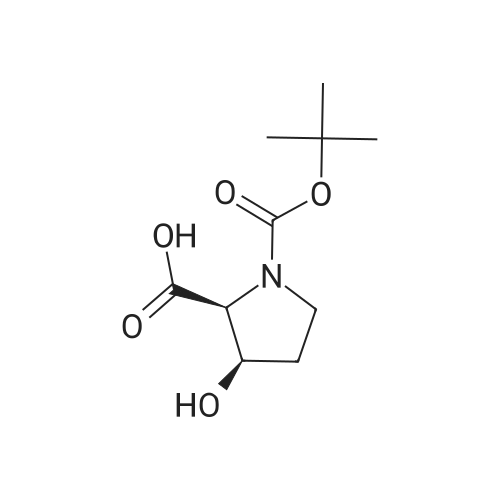
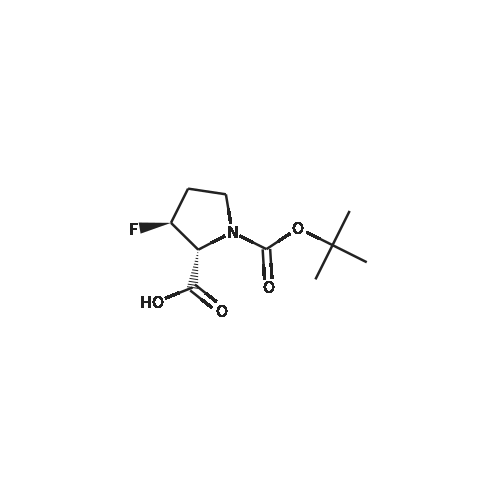
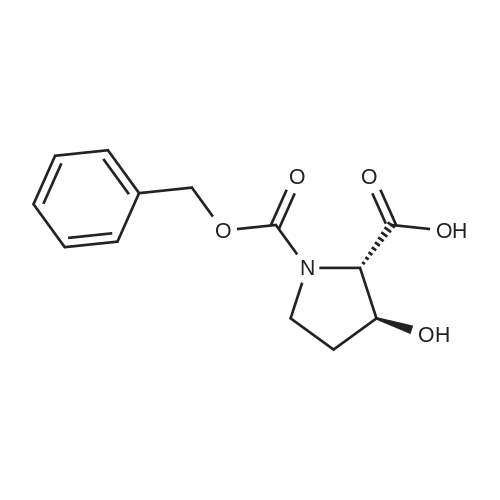
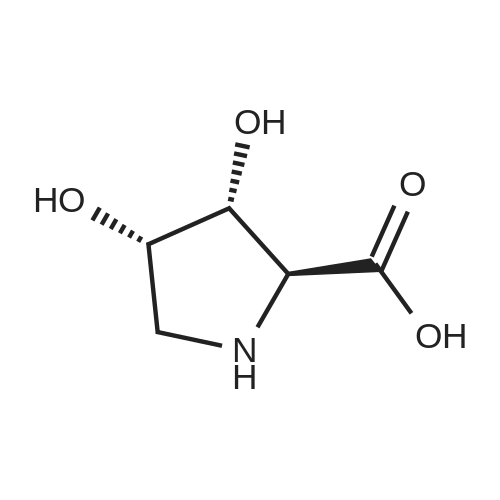
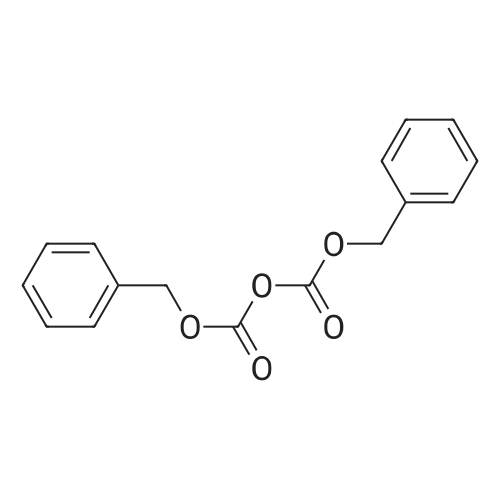
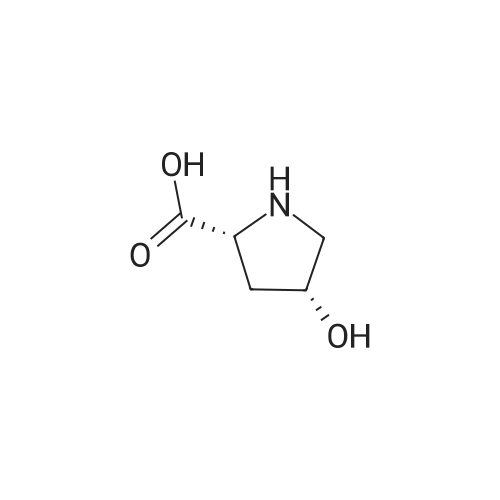
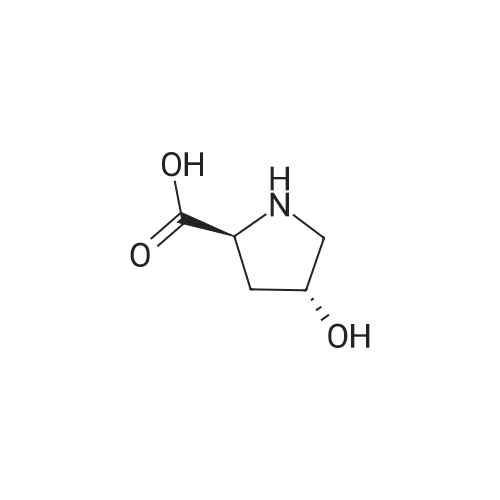
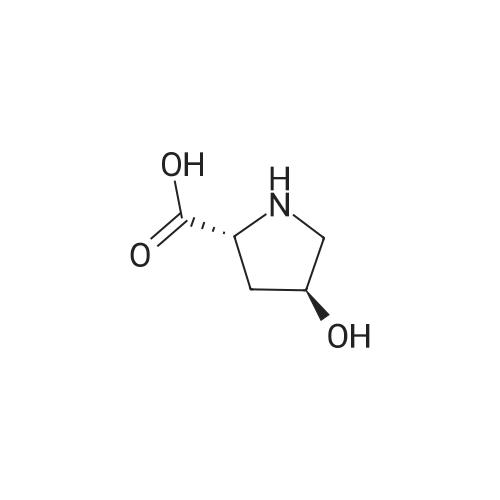
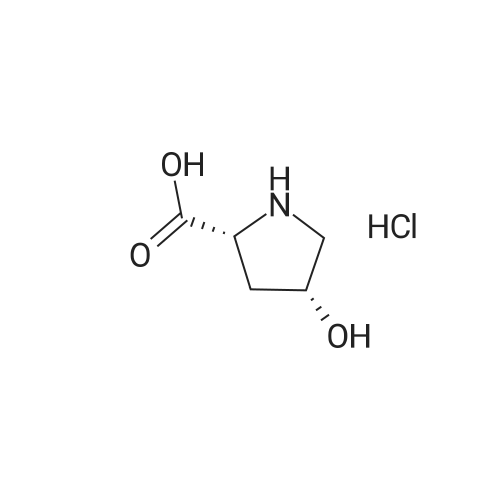
| ~ 85% |
With sodium carbonate; In 1,4-dioxane; water; at 20℃; for 1.5h; |
Boc anhydride (2.95 g, 13.5 mmol) was added to a stirred solution of the (2S, 3S)-3-HYDROXYPYRROLIDINE-2-CARBOXYLIC acid (1.61 g, 12.3 mmol) and sodium carbonate (1.3 g, 12.3 mmol) in a mixture of dioxane (25 ml) and water (12.3 ml). The mixture was stirred for 1.5 h at ambient temperature then evaporated under reduced pressure to afford a residue (-10 ml). The residue was diluted with water (30 ML) then extracted with ethyl acetate (40 ml). The aqueous phase was acidified (PH-2. 5) with dilute aqueous hydrochloric acid (0.1 M) then extracted with chloroform (4 x 50 ml). The combined organic layers were dried (NA2SO4) and evaporated under reduced pressure to afford (2S, 3S)-3-hydroxypyrrolidine-1, 2-dicarboxylic acid 1-TERT-BUTYL ester as a white crystalline solid (2.39 G,-85%), HPLC- MS (single main peak, 254.1 [M + Na] + and 485.2 [M + H] +). |
| 70% |
With sodium hydroxide; In tetrahydrofuran; water; |
EXAMPLE 18A (2S,3 S)-1-(tert-butoxycarbonyl)-3-hydroxy-2-pyrrolidinecarboxylic acid trans-3-Hydroxy (L)-proline (10.0 g, 76.3 mmol) in THF (50 mL) was treated with sodium hydroxide (3.36 g, 84 mmol) in water (34 mL) at ambient temperature. After 10 minutes of stirring, the mixture was treated with di-tert-butyl dicarbonate (16.63 g, 76.3 mmol) portionwise. After stirring at ambient temperature for 10 hours, the mixture was concentrated under reduced pressure, acidified to pH 2-3 with saturated KHSO4 (aq), and extracted with ethyl acetate (2*200 mL). The organic extracts were combined, washed with brine (2*30 mL), and concentrated to provide the title compound as a white solid (12.3 g, 70%, yield). mp 156-157 C. |
| 70% |
|
trans-3-Hydroxy-(L)-proline (10.0 g, 76.3 mmol) in THF (50 mL) was treated with sodium hydroxide (3.36 g, 84 mmol) in H2O (34 mL) and then treated with di-tert-butyl dicarbonate (16.63 g, 76.3 mmol) portionwise. After stirring at ambient temperature for 10 hours, the mixture was concentrated under reduced pressure to remove the THF. The residue was acidified to pH 2-3 with saturated KHSO4 and extracted with ethyl acetate (2 x 200 mL). The organic extracts were combined, washed with brine (2 x 30 mL) and concentrated to provide the title compound as a white solid (12.3 g, 70%). mp 156-157 C. |
|
With sodium hydroxide; In 1,4-dioxane; water; at 5 - 20℃; for 16.5h; |
A mixture of trans-3-hydroxy-L-proline (5 g), di-tert-butyl dicarbonate (9.15 g), sodium hydroxide (1.52 g), water (78 ml) and dioxan (80 ml) was stirred at 5 C. for 30 mins. and then at ambient temperature for 16 hours. The mixture was evaporated to a smaller volume (30 ml) and diluted with water (150 ml). The pH was adjusted to 2-3 with aqueous sodium bisulphate and saturated with sodium chloride. It was then extracted with ethyl acetate (3*100 ml), the extracts dried and the solution evaporated to dryness to give (2S,3S)-1-(tert-butoxycarbonyl)-2-carboxy-3-hydroxypyrrolidine (compound 64) as a white solid (8.42 g). Compound 64: NMR (DMSOd6) delta: 1.27 (2s, 9H), 1.64-1.76 (m, 2H), 3.24-3.45 (m, 2H), 3.92 (d, 1H), 4.20 (br, 1H), 5.40 (br, 1H), 12.6 (br, 1H). |
|
With sodium hydrogencarbonate; In tetrahydrofuran; water; at 0 - 20℃; for 16h; |
To a cold (0 C) mixture of (2S,3S)-3-hydroxypyrrolidine-2-carboxylic acid 26a (6.37g, 48.6 mmol) in tetrahydrofuran (180 mL) is added di-tert-butyl dicarbonate (26.4 g, 0.121 mmol) followed by addition of solution of NaHCO3 (24.5 g, 0.292 mmol) in water (180 mL). The cooling bath is removed and the mixture is stirred 16 hours at room temperature. The solvent is evaporated in vacuo, the residue is diluted with water and extracted with ether. The ethereal layer is discarded. The aqueous layer is cooled to 0 C, acidified to pH 1 with 6 N hydrochloric acid, extracted with dichloromethane (3x) and n-butanol (2x). The combined organic phase is concentrated in vacuo. The oily residue is co-evaporated twice with toluene anddried on a high vacuum pump to give (2S,3S)-1-(tert-butoxycarbonyl)-3-hydroxypyrrolidine-2-carboxylic acid B16b: ESI-MS calcd. for C10H17NNaO5 ([M+Na]+) 254.1, found 254.1. The product is used without further purification. |
| 0.91 g |
With N-ethyl-N,N-diisopropylamine; In dichloromethane; at 20℃; |
To a solution of (2S,3S)-3-hydroxypyrrolidine-2-carboxylic acid (0.50 g, 3.8 mmol) in CH2Cl2 (19 mL) at roomtemperature was added di-tert-butyl dicarbonate (0.96 mL, 4.2 mmol) and Hunig?s base (1.32 mL, 7.6 mmol) to give atan solution. The reaction was stirred overnight at room temperature and then diluted with CH2Cl2 and washed withsaturated sodium bicarbonate solution. The aqueous layer was concentrated under reduced pressure and then suspendedin methanol and filtered. The solvent was then removed under reduced pressure to prodive the title compound(0.91g, 103% yield). |
|
With water; sodium hydroxide; In 1,4-dioxane; at 20℃; for 2h; |
Step 1 : (2S,3S)-l-(tert-Butoxycarbonyl)-3-hvdroxypyrrolidine-2-carboxylic acid.To a solution of (2S,3S)-3-hydroxypyrrolidine-2-carboxylic acid (3.0 g, 23 mmol) in dioxane/H20 (40 mL/20 mL) was added sodium hydroxide (1.83 g, 46 mmol), followed by the dropwise addition of di-tert-butyl dicarbonate (9.49 g, 43 mmol) in dioxane (15 mL). The reaction mixture was stirred at room temperature (rt) for 2 h. The reaction mixture was extracted with EtOAc (50 mL) and the organic layer was washed with 10% aq. NaOH (30 mL). The combined aqueous layers were acidified with cone. HC1 to pH 2 and extracted with CH2C12. The organic phases were dried (Na2S04) and concentrated to give the crude product, which was used without further purification (5.01 g, 95%). 1H NMR (CD3OD, 400 MHz) delta 4.40- 4.30 (m, 1H), 4.15-4.00 (m, 1H), 3.50-3.40 (m, 2H), 2.00-1.95 (m, 1H), 1.85 (m, 1H), 1.45- 1.35 (m, 9H). |
|
With sodium hydrogencarbonate; In 1,4-dioxane; water; |
(2S,3S)-1-(Tert.-butoxycarbonyl)-3-hydroxypyrrolidine-2-carboxylic acid (15) was prepared by Boc protection of the secondary amino group of 13 by di-tert. -butyl dicarbonate in dioxane in the presence of aq. Na2CC>3 soln applying standard conditions. Data of 15: Ci0Hi7NO5 (231 .2). FI-MS: 230.1 ([M-H]-). 1H-NMR (DMSO-d6): 5.43 (br. s, 1 H); 4.23 (br. m, 1 H); 3.92 (d, J = 12.4, 1 H); 3.46 - 3.29 (m, 2 H); 1 .85 (m, 1 H); 1 .72 (m, 1 H); 1 .40, 1.34 (2 s, 9 H). |
|
|
To a stirred solution of (2 S, 3S)-3-hydroxypyrrolidine-2-carboxylic acid (3.00 g, 22.88 mmol) in THF (150 mL) was added water (150 ml_) and NaHCCb (7.70 g, 92 mmol) at 0 C. The reaction mixture was stirred at room temperature for 15 minutes. After B0C2O (7.50 g, 34.4 mmol) was added, the reaction mixture was stirred at 25 C for 16 h. The resulting mixture was added sat'd aqueous NaHCC>3 (50 mL) and washed with ethyl ether (2 x 100 mL). The separated organic phases were deserted. The pH value of the aqueous phase was adjusted to 3 with aqueous HCI (1 M). The aqueous solution was extracted with EA (6 x 200 mL). The organic layers were combined and concentrated under reduced pressure to afford 5.00 g (90% yield) of 123 as an off-white solid. LCMS (ESI) calc?d for C10H17NO5 [M + Na]+: 254.1 , found 253.9. 1H NMR (300 MHz, CDsOD) d 4.47-4.33 (m, 1 H), 4.19-4.08 (m, 1 H), 3.69-3.44 (m, 2H), 2.16-1.97 (m, 1 H), 1.94-1.81 (m, 1 H), 1.45 (d, J = 13.3 Hz, 9H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping