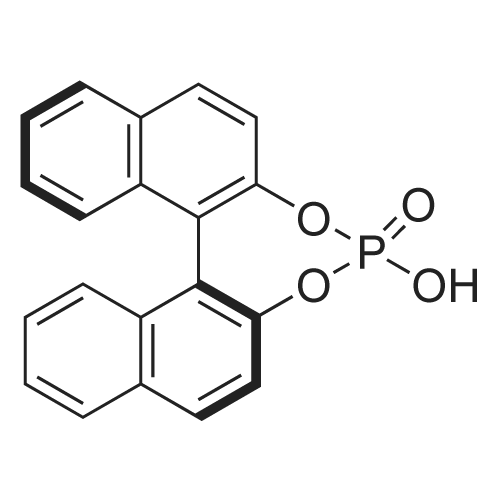
Alternatived Products of [ 39648-67-4 ]
Product Details of [ 39648-67-4 ]
| CAS No. : | 39648-67-4 |
MDL No. : | MFCD00010045 |
| Formula : |
C20H13O4P
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | - |
| M.W : |
348.29
|
Pubchem ID : | - |
| Synonyms : |
|
Chemical Name : | (R)-4-Hydroxydinaphtho[2,1-d:1',2'-f][1,3,2]dioxaphosphepine 4-oxide |
Application In Synthesis of [ 39648-67-4 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 39648-67-4 ]
- 1
-
 [ 39648-67-4 ]
[ 39648-67-4 ]

-
 [ 102625-70-7 ]
[ 102625-70-7 ]

-
 [ CAS Unavailable ]
[ CAS Unavailable ]
| Yield | Reaction Conditions | Operation in experiment |
| 98 % ee |
With sodium hydroxide In cyclohexane; benzene at 50 - 55℃; for 0.5h; |
14
Example 14: Preparation of S-pantoprazole-(R)-BNPPA complex; R-l ,l'-binaphthyl-2-2'-diyl hydrogen phosphate (1 1 gm, 0.031 moles) was added to a solution of 5-(difluoromethoxy)-2-[[(3,4-dimethoxy-2-pyridinyl)methyl]sufinyl]-l H- benzimidazole (pantoprazole) (10 gm, 0.026 moles) and powder sodium hydroxide (3 gm) in a mixture of benzene and cyclohexane (250 ml, 1 :1.5) at room temperature and heated to 50 to 55°C for 30 minutes. Then the solution was cooled to 40°C, filtered. The solid obtained was washed with a mixture of benzene and cyclohexane (40 ml, 1 :1 ), and then dried at 45 to 50 deg C under vacuum for 4 hours to obtain 4.5 gm of S- pantoprazole-(R)-BNPPA complex (chiral purity: 98%). |
| 98 % de |
With sodium hydroxide In cyclohexane; benzene at 20 - 55℃; for 0.5h; |
14
R-1,1'-binaphthyl-2-2'-diyl hydrogen phosphate (11 gm, 0.031 moles) was added to a solution of 5-(difluoromethoxy)-2-[[(3,4-dimethoxy-2-pyridinyl)methyl]sufinyl]-1H-benzimidazole (pantoprazole) (10 gm, 0.026 moles) and powder sodium hydroxide (3 gm) in a mixture of benzene and cyclohexane (250 ml, 1:1.5) at room temperature and heated to 50 to 55° C. for 30 minutes. Then the solution was cooled to 40° C., filtered. The solid obtained was washed with a mixture of benzene and cyclohexane (40 ml, 1:1), and then dried at 45 to 50 deg C. under vacuum for 4 hours to obtain 4.5 gm of S-pantoprazole-(R)-BNPPA complex (chiral purity: 98%). |
- 2
-
 [ 35193-63-6 ]
[ 35193-63-6 ]

-
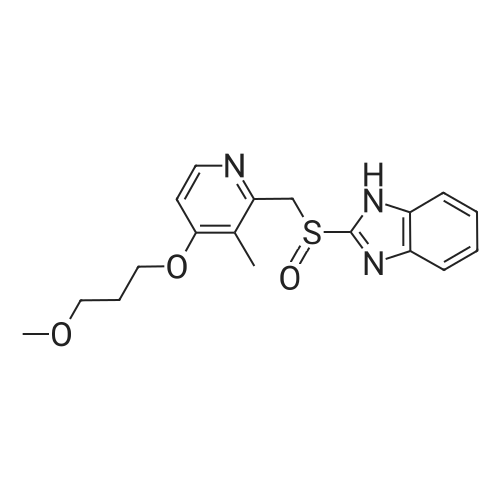 [ 117976-89-3 ]
[ 117976-89-3 ]

-
(S)-2-[[[4-(3-methoxy-propoxy)-3-methyl-2-pyridinyl]methyl]sulfinyl]-1H-benzimidazole R-binaphthyl-2-2'-diyl hydrogenphosphate
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
With sodium hydroxide; In cyclohexane; benzene; at 50 - 55℃; for 0.5h; |
Example 15:Preparation of S-<strong>[117976-89-3]rabeprazole</strong>-(R)-BNPPA complex; R-l,l'-binaphthyl-2-2'-diyl hydrogen phosphate (12 gm, 0.034 moles) was added to a solution of 2-[[[4-(3-methoxy-propoxy)-3-methyl-2-pyridinyl]methyl]sulfinyl]-l H- benzimidazole (<strong>[117976-89-3]rabeprazole</strong>) (10 gm, 0.028 moles) and powder sodium hydroxide (3 gm) in a mixture of benzene and cyclohexane (250 ml, 1 : 1.5) at room temperature and heated to 50 to 55C for 30 minutes. Then the solution was cooled to 40C, filtered. The solid obtained was washed with a mixture of benzene and cyclohexane (40 ml, 1 : 1), and then dried at 45 to 50 deg C under vacuum for 4 hours to obtain 4.7 gm of S-<strong>[117976-89-3]rabeprazole</strong>-(R)- BNPPA complex (chiral purity: 98.2%). |
|
With sodium hydroxide; In cyclohexane; benzene; at 20 - 55℃; for 0.5h; |
R-1,1'-binaphthyl-2-2'-diyl hydrogen phosphate (12 gm, 0.034 moles) was added to a solution of 2-[[[4-(3-methoxy-propoxy)-3-methyl-2-pyridinyl]methyl]sulfinyl]-1H-benzimidazole (<strong>[117976-89-3]rabeprazole</strong>) (10 gm, 0.028 moles) and powder sodium hydroxide (3 gm) in a mixture of benzene and cyclohexane (250 ml, 1:1.5) at room temperature and heated to 50 to 55 C. for 30 minutes. Then the solution was cooled to 40 C., filtered. The solid obtained was washed with a mixture of benzene and cyclohexane (40 ml, 1:1), and then dried at 45 to 50 deg C. under vacuum for 4 hours to obtain 4.7 gm of S-<strong>[117976-89-3]rabeprazole</strong>-(R)-BNPPA complex (chiral purity: 98.2%). |
- 3
-
 [ 39648-67-4 ]
[ 39648-67-4 ]

-
 [ 298-59-9 ]
[ 298-59-9 ]

-
 [ 312960-65-9 ]
[ 312960-65-9 ]
| Yield | Reaction Conditions | Operation in experiment |
| 27% |
With 4-methyl-morpholine In methanol; water at 20 - 50℃; for 4h; Resolution of racemate; |
|
- 4
-
 [ 39648-67-4 ]
[ 39648-67-4 ]

-
 [ 298-59-9 ]
[ 298-59-9 ]

-
 [ 312960-65-9 ]
[ 312960-65-9 ]

-
 [ 312960-66-0 ]
[ 312960-66-0 ]
| Yield | Reaction Conditions | Operation in experiment |
| 56.4 % ee |
With 4-methyl-morpholine In methanol; water at 20 - 50℃; for 3h; Resolution of racemate; Overall yield = 24.1 %; |
|
- 5
-
 [ 39648-67-4 ]
[ 39648-67-4 ]

-
 [ 50-41-9 ]
[ 50-41-9 ]

-
 [ 1809294-13-0 ]
[ 1809294-13-0 ]
| Yield | Reaction Conditions | Operation in experiment |
| 76.4 g |
In methanol at 20 - 45℃; for 1h; |
2d Preparation of Salt of Trans-Clomiphene With Racemic Binaphthyl-Phosphoric, Starting From Clomiphene Citrate
Preparation of Salt of Trans-Clomiphene With Racemic Binaphthyl-Phosphoric, Starting From Clomiphene Citrate In a round bottom flask was charged 150 gr of Clomiphene Citrate (the same as that used in example 2a) and 1500 mL of Methanol. The suspension was heater at 40-45° C. and stirred up the complete dissolution. Then a solution of BPA 45 gr (0.515 eq) in 900 mL of methanol was added. At the end of addition the mixture was stirred for 1 hour at 20° C. The obtained suspension was filtered and the solid was washed with 100 mL of methanol. 76.4 gr of E-Clomiphene BPA salt were obtained. |
| 47.9 g |
In methanol at 20 - 45℃; for 1h; |
1c; 2 Example 1 c: Preparation of salt of Enclomiphene with racemic binaphthyl- phosphoric acid, starting from Clomiphene citrate.
In a round bottom flask was charged 100 gr of Clomiphene Citrate and 1000 ml_ of methanol. The suspension was heated at 40-45°C and stirred up the complete dissolution. Then a solution of BPA 30 gr (0.515 eq) in 1000 ml_ of methanol was added. At the end of addition the mixture was stirred for 1 h at 20°C. the obtained suspension was filtered and the solid was wash with 100 ml_ of methanol. 47.9 gr of Enclomiphene BPA salt were obtained. HPLC Analysis (A/A%): 98.81 % Enclomiphene, 0.79% Z-Clomiphene. Recrystallization of Enclomiphene BPA salt of formula (III) (the step A). [00201] Into a proper 0.5 L reactor, equipped with propeller, temperature probes, condenser; Enclomiphene BPA salt (III) (50 g) and having Z-isomer of 1.64 % was suspended in DMF (2.1 L/Kg of Enclomiphene BPA (III)) and methanol (1.4 L/Kg of Enclomiphene BPA salt (III)). The suspension was heated to reflux (~ 76-79°C). Further DMF (0.1 L/Kg of Enclomiphene BPA (III)) might be required to improve the solubility of the starting material. Once the starting material was completely dissolved, methanol was added as anti-solvent (3.5 L/Kg of Enclomiphene BPA (III)). The temperature was decreased to 60°C and the mixture was stirred for 2 - 3 h. Then, the temperature was further decreased to 20 °C and filtered. The wet cake was washed twice with methanol (1.5 L/Kg of Enclomiphene BPA salt (III)). The product was dried under vacuum at 60 - 70 °C for 12 - 24 h. Time of drying could be prolonged until residual DMF is < 2500 ppm. Analysis of quality of the final product of the above mentioned example and of the same product, obtained from repetition following the same process, it is shown in the following table: Enclomiphene BPA (III) salt Enclomiphene BPA (III) salt rixx (Starting product) (finale product) Z-isomer = 1.64 A/A% Z-isomer = 0.07 A/A% Z-isomer = 0.79 A/A% Z- isomer = 0.03 A/A% |
| 7.3 g |
In methanol at 45 - 50℃; for 4h; |
1 Comparative Example 1: Preparation of Zuclomiphene Citrate Using Conditions Analogous to U.S. Pat. No. 3,848,030 A
Methanol (35 mL) was added to a mixture of clomiphene citrate ((1)(citric acid), E:Z isomeric ratio 60:40, 10 g, 0.0167 mol) and racemic binaphthyl hydrogen phosphate (5.82 g, 0.0167). Initially, a clear brown solution was obtained, which became turbid after about 10 minutes and then formed a thick paste which stuck to the flask walls; heating the thick paste to 45-50° C. for about one hour did not affect the consistency. Methanol (20 mL) was charged and heating was continued for an additional hour, but the reaction mixture remained as a creamy paste. Heating was discontinued and the mixture was stirred for two hours, however the consistency of the material remained unchanged. The unpourable material was scooped out of the flask and the flask was washed with methanol (4×10 mL) to facilitate effective transfer. The damp cake was dried in vacuo at 50-60° C. for about 18 hours to afford solid enclomiphene binaphthyl hydrogen phosphate ((1-B)(BPA), 7.30 g, 96.5% recovery of available) as a white to off-white solid. The solid was determined to have an isomeric purity of about 94.6% (E) by 1H NMR. |
- 6
-
 [ 39648-67-4 ]
[ 39648-67-4 ]

-
 [ 911-45-5 ]
[ 911-45-5 ]

-
 [ 1809294-13-0 ]
[ 1809294-13-0 ]
| Yield | Reaction Conditions | Operation in experiment |
|
In methanol at 20℃; for 2h; |
1 Separation of Clomiphene Isomers
Clomiphene (8) obtained above is dissolved in methanol and racemic binaphthyl-phosphoric acid (BPA) is added under stirring. When the precipitate begins separating from the solution, stirring is stopped and the mixture is allowed to settle at room temperature for 2 hours. The precipitate is filtered, washed with methanol and ether and dried. Trans-clomiphene-BPA salt (3) is obtained. |
- 7
-
 [ 39648-67-4 ]
[ 39648-67-4 ]

-
 [ 50-41-9 ]
[ 50-41-9 ]

-
 [ 39648-80-1 ]
[ 39648-80-1 ]
| Yield | Reaction Conditions | Operation in experiment |
| 7.3 g |
In methanol; tert-butyl methyl ether at 20 - 55℃; for 23h; |
3 Example 3: Preparation of Solid Zuclomiphene Binaphthyl Hydrogen Phosphate Salt (1-A)(BPA)
A solution of clomiphene citrate ((1)(citric acid), E:Z isomeric ratio 60:40, 20 g, 0.0334 mol) and racemic binaphthyl hydrogen phosphate (BPA) (12.2 g, 0.0351) in methanol (280 mL) was stirred at room temperature. The resulting suspension was heated to 50-55° C. for about five hours to afford a uniform mixture. The mixture was cooled to room temperature and stirred for about three hours prior to filtration at room temperature, washing with methanol (1×20 mL), and drying in vacuo at 40-45° C. for about 18 hours to afford solid enclomiphene binaphthyl hydrogen phosphate ((1-B)(BPA), 14.09 g, 93.1% recovery of available). The solid was determined to have an isomeric ratio of enclomiphene: zuclomiphene of about 97:3 (97% isomeric purity (E)) by 1H NMR. (0153) The resulting mother liquor was concentrated to about 100 mL, methyl t-butyl ether (80 mL) was added and the resulting mixture was stirred at room temperature for about 18 hours. The resulting suspension was stirred at 0-5° C. for about four hours prior to filtration and drying in vacuo at 45-50° C. to afford solid zuclomiphene binaphthyl hydrogen phosphate ((1-A)(BPA), 7.3 g, 72.4% recovery of available). The solid was determined to have an isomeric ratio of zuclomiphene:enclomiphene of about 97:3 (97% isomeric purity (Z)) by 1H NMR. |
- 8
-
 [ 39648-67-4 ]
[ 39648-67-4 ]

-
 [ 1170736-59-0 ]
[ 1170736-59-0 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1.1: sec.-butyllithium / tetrahydrofuran; hexane / 2 h / -78 °C / Inert atmosphere
1.2: Ca. 150 min / 20 °C / Inert atmosphere
2.1: potassium <i>tert</i>-butylate; potassium dihydrogenphosphate; 4,7-dimethoxy-1,10-phenanthroline; (TMEDA)Ni(o-tolyl)Cl / butan-1-ol / 16 h / 60 °C / Inert atmosphere |
|

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping