| 235 mg |
With N-ethyl-N,N-diisopropylamine In dimethyl sulfoxide at 100℃; for 16h; |
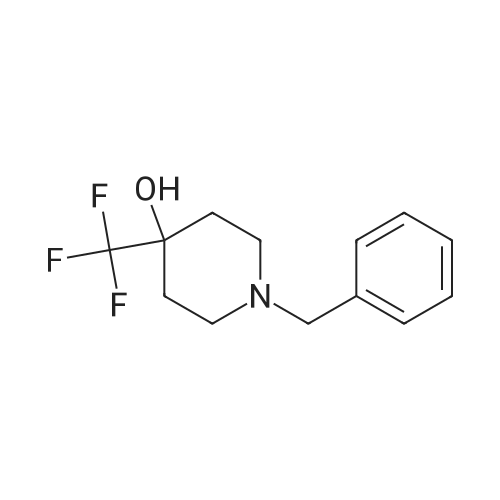
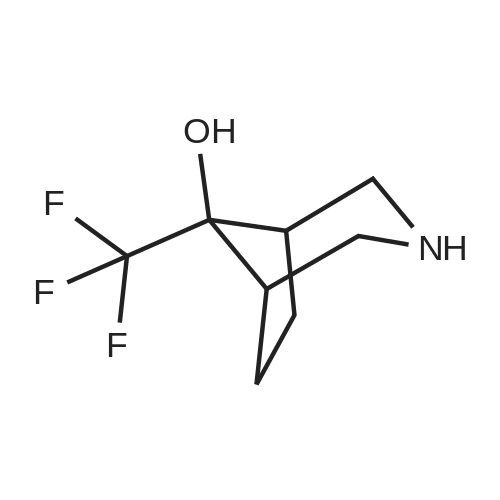
11-2-1; 11-2-2 Examples 11-2-1 and 11-2-2: 1-(2-(8,0-Dimet yltetrahydro-2W-pyran-3-yl)-5- iTiethyfphenoxy)-f -((6-(4- ydroxy-4-(tnfluoromet yi)pspendin-1-yl)pyridin-2- yl)sulfonyS)cycloproparie-1 -carboxamide, Enantiomer 1 PEAK 1 and Enantiomer 2
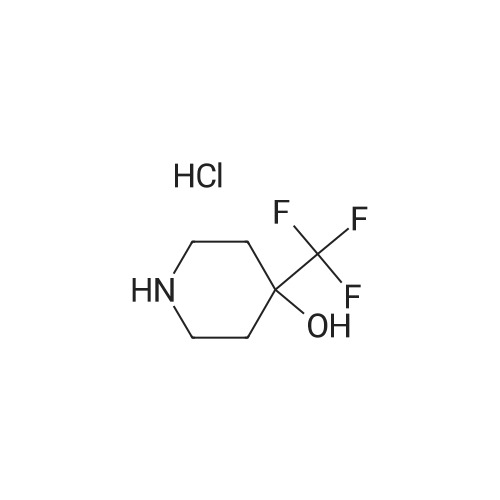
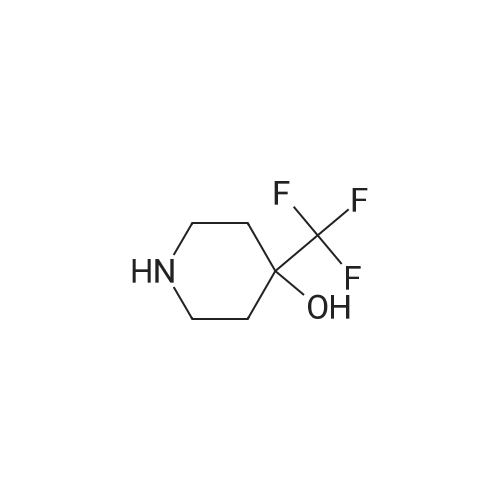
To the stirred solution of 1-(2-(6,6-dimeihylteirahydro-2/-/-pyran-3-yl)-5-methylphenoxy)-/V- ((6~iluoropyridin-2~yl)suifonyi)cycopropane-1 -carboxamide (I 29-1) (300 mg, 0.6 mmol) in DMSO (2 mL), 4-{trifuoromeihy)piperidin~4~o (274 mg, 1.6 mmol) and DIPEA (90 pL, 0.5 mmol) were added at rt. The reaction mixture was stirred at 100 °C for 16 h. The reaction mixture was quenched with saturated aqueous citric acid solution, extracted with (1460) dichloromethane twice. The combined organic extracts were washed with water, brine and dried over anhydrous Na S0 , filtered and concentrated in vacuo. The crude product was purified by reversed phase HPLC [Kinetex EVO C18 (250 mm x 21.20 mm), 5 micron, 20 mL/min, 20-50% acetonitriie/0.05% formic acid in water] to obtain the racemic mixture of 1- (2-(6,6-Dimethyltetrahydro-2H-pyran-3-yl)-5-methylphenoxy)-//-((6-(4-hydroxy-4- (trifluoromethyi)piperdn-1-yl)pyridin-2-yl)sulfonyl)cyclopropane-1 -carboxamide as a while solid (235 mg, 59% yield). The racemic mixture was subjected to chiral normal-phase HPLC [Chiralpak IG (25Q mm 1Q mm), 5 micron, isocraiic 20% (0.2% formic acid in 1 :1 (1461) EtOH/MeOH)/hexane] and yielded the corresponding enantiomers: Enantiomer 1, Ex.11-2- 1 , Rt = 8.680 min under chiral reverse-phase HPLC (Lux, Cellulose-4, 250 x 4.8 mm, 5 micron; isocractic 70:30 hexane/(0.1% formic acid in 1:1 EtOH/MeOH); 10 mL/rnin). (1462) Condition 3, LCMS: rn/z 612.3 [M]+, 0.70 min.1H NMR (400 MHz, Methanol-*) d [7.73 (dd, J= 8.8, 7.2 Hz, 1 H), 7.29 (d, J= 7.2 Hz, 1H), 7.14 (d, J= 8.0 Hz, 1H), 7.09 (d, J= 8.4 Hz, 1H), 8.80 (d, J= 7.6 Hz, 1 H), 6.54 (s, 1 H), 4.29 (d, J= 12.8 Hz, 2H), 3.83 - 3.80 (m, 1 H), 3.53 (t, (1463) J= 10.8 Hz, 1 H), 3.21 -3.12 (m, 3H), 2.18 (s, 3H), 2.00- 1.92 (m, 1H), 1.77- 1.74 (m, 4H), (1464) 1.71 - 1.63 (m, 3H), 1.55 - 1.50 (m, 1 H), 1.48 - 1.43 (m, 1 H), 1.30 (s, 3H), 1.25 - 1.22 (m, 4H), 1.17 - 1.14 (m, 1H).j. Enantiomer 2, Ex.11-2-2, Rt = 10.382 min under chiral reverse- phase HPLC (Lux, Cellulose-4, 250 x 4.6 mm, 5 micron; isocractic 70:30 hexane/(Q.1% formic acid in 1:1 EtOH/MeOH); 1.0 mL/min). Condition 3, LCMS: m/z 612.3 [M]+, 0.70 min. 1H NMR (4QQ MHz, Methanol-*) d 7.73 (dd, J= 9.2, 7.8 Hz, 1H), 7.29 (d, J= 7.6 Hz, 1H), (1465) 7.14 (d, J= 8.Q Hz, 1H), 7.08 (d, J= 8.0 Hz, 1H), 8.80 (d, J= 7.6 Hz, 1H), 6.54 (s, 1H), 4.29 (d, J= 13.2 Hz, 2H), 3.63-3.60 (m, 1H), 3.53 (t, J= 10.8 Hz, 1H), 3.19-3.12 (m, 3H), 2.18 (s, 3H), 1.98 - 1.92 (m, 1 H), 1.77 - 1.74 (m, 4H), 1.71 -1.64 (m, 3H), 1.56 - 1.51 (m, 1 H), 1.48 -1.43 (m, 1 H), 1.30 (s, 3H), 1.25-1.23 (m, 4H), 1.17-1.12 (m, 1H).J. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping