Alternatived Products of [ 356783-42-1 ]
Product Details of [ 356783-42-1 ]
| CAS No. : | 356783-42-1 |
MDL No. : | MFCD18968605 |
| Formula : |
C5H5FN4O
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | - |
| M.W : |
156.12
|
Pubchem ID : | - |
| Synonyms : |
|
Safety of [ 356783-42-1 ]
| Signal Word: | |
Class: | N/A |
| Precautionary Statements: | |
UN#: | N/A |
| Hazard Statements: | |
Packing Group: | N/A |
Application In Synthesis of [ 356783-42-1 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 356783-42-1 ]
- 1
-
 [ 79-37-8 ]
[ 79-37-8 ]

-
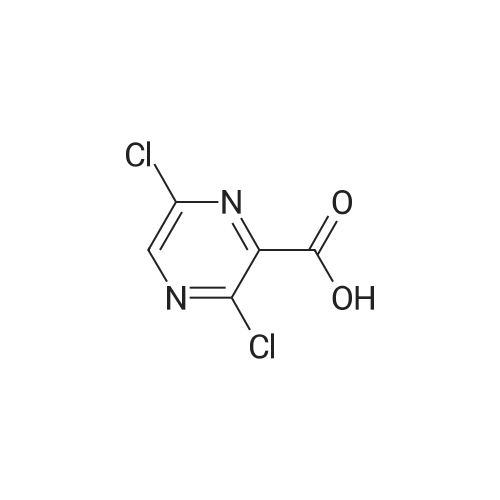 [ 356783-15-8 ]
[ 356783-15-8 ]

-
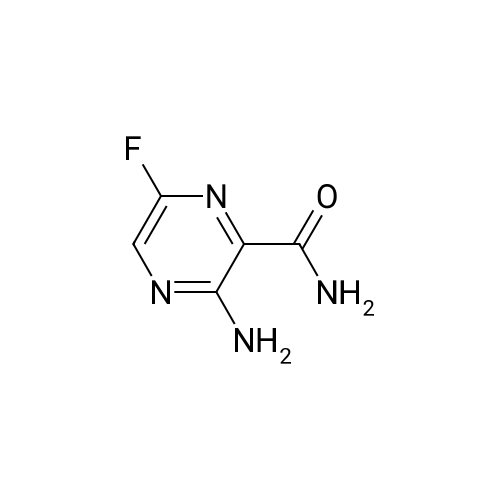 [ 356783-42-1 ]
[ 356783-42-1 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With ammonium hydroxide; potassium fluoride; sodium chloride;18-crown-6 ether; In dichloromethane; water; ethyl acetate; N,N-dimethyl-formamide; acetonitrile; |
(b) In 2.0 mL of methylene chloride was suspended 0.2 g of <strong>[356783-15-8]3,6-dichloro-2-pyrazinecarboxylic acid</strong>. Then, 0.001 mL of N,N-dimethylformamide and 0.14 mL of oxalyl chloride were successively added at an ice-cooled temperature, and the mixture thus formed was stirred at room temperature for one hour. The reaction mixture was concentrated to dryness under reduced pressure, the residue was dissolved in 3.0 mL of acetonitrile, 0.35 g of potassium fluoride and 0.054 g of 18-crown-6-ether were added, and the mixture thus obtained was stirred at 60 C. for 3 hours. Then, 3.0 mL of 25% aqueous ammonia was added to the reaction mixture at room temperature, and the mixture thus obtained was stirred at 50 C. for 2.5 hours. The reaction mixture was poured into a mixture of 30 mL of ethyl acetate and 30 mL of water, and the organic layer was separated. The organic layer thus obtained was washed successively with 15 ml of water and 15 ml of saturated aqueous solution of sodium chloride and dried on anhydrous magnesium sulfate, and the solvent was removed under reduced pressure. The deposited product was washed with diethyl ether, and there was obtained 0.12 g of 3-amino-6-fluoro-2-pyrazinecarboxamide as a yellow-colored solid product. Physical properties of this compound coincided with those of the compound obtained in Example II-11(a). |
- 2
-
 [ 356783-42-1 ]
[ 356783-42-1 ]

-
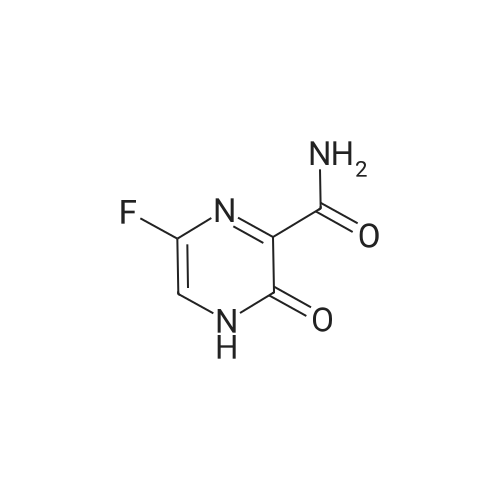 [ 259793-96-9 ]
[ 259793-96-9 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With sodium bicarbonate; sodium chloride; sulfuric acid; sodium nitrite; In (2S)-N-methyl-1-phenylpropan-2-amine hydrate; di-isopropyl ether; water; ethyl acetate; |
PRODUCTION EXAMPLE 3 While keeping 285 ml of 97% sulfuric acid at 5-12 C. by cooling it with ice, 28.5 g of 3-amino-6-fluoro-2-pyrazinecarboxamide was added thereto to form a uniform solution. After adding 18.9 g of sodium nitrite to the solution at 5-12 C., the mixture thus obtained was stirred for 1.5 hours while cooling it with ice. While keeping the reaction mixture at a temperature not exceeding 10 C., the reaction mixture was dropwise added to 1.4 L of ice water, and the mixture thus formed was extracted first with one 850 mL portion and then two 200 mL portions of ethyl acetate. The organic layers thus obtained were united, 400 mL of water was added, then 160 mL of saturated aqueous solution of sodium hydrogen carbonate was added, pH was adjusted to 3.0, and the organic layer was separated. The organic layer thus obtained was washed with saturated aqueous solution of sodium chloride and dried on anhydrous magnesium sulfate, and the solvent was removed under reduced pressure. The residue thus obtained was washed with a mixture of diisopropyl ether and ethyl acetate to obtain 22.4 g of 6-fluoro-3-hydroxy-2-pyrazinecarboxamide as a solid product. |
- 3
-
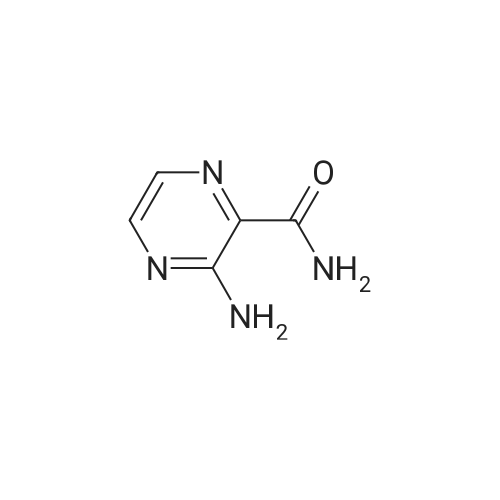 [ 32587-10-3 ]
[ 32587-10-3 ]

-
 [ 356783-42-1 ]
[ 356783-42-1 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With sodium bicarbonate; sodium chloride; fluorine; In water; ethyl acetate; trifluoroacetic acid; |
(c) In 9 mL of trifluoroacetic acid was dissolved 0.3 g of <strong>[32587-10-3]3-amino-2-pyrazinecarboxamide</strong>. At an ice-cooled temperature, 10% fluorine gas (a fluorine gas diluted with nitrogen gas) was introduced into the solution at a rate of 45 ml per minute for a period of 22 minutes. After stirring the mixture at an ice-cooled temperature for 17 minutes, the temperature was elevated to room temperature. The reaction mixture was added to a mixture of 30 mL of saturated aqueous solution of sodium hydrogen carbonate and 30 mL of ethyl acetate, and the organic layer was separated. The remaining aqueous layer was acidified with 6 mol/L hydrochloric acid and then extracted with 20 ml of ethyl acetate. The organic layers thus obtained were united, washed successively with 10 mL of water and 10 mL of saturated aqueous solution of sodium chloride and dried on anhydrous magnesium sulfate, and the solvent was removed under reduced pressure. The residue thus obtained was purified by silica gel column chromatography [eluent: n-hexane:ethyl acetate=2:1] to obtain 0.015 g of 3-amino-6-fluoro-2-pyrazinecarboxamide as a light yellow-colored solid product. |
|
With nitrogen; fluorine; In water; trifluoroacetic acid; |
(d) In 5 mL of trifluoroacetic acid was dissolved 100 mg of <strong>[32587-10-3]3-amino-2-pyrazinecarboxamide</strong>. At an ice-cooled temperature, 10% fluorine gas (a fluorine gas diluted with nitrogen gas) was introduced at a rate of 45 mL per minute for a period of 36 minutes. Then, while elevating the temperature from the ice-cooled temperature to room temperature, nitrogen gas was introduced for one hour. The reaction mixture was concentrated under reduced pressure to obtain 305 mg of an oily product. Of the oily product thus obtained, a 251 mg portion was dissolved in 9.3 mL of water and heated under reflux for 4 hours, The liquid reaction mixture was cooled to room temperature, and the deposited precipitate was filtered off. The filtrate was concentrated under reduced pressure, and the solid product thus obtained was purified by silica gel column chromatography [eluent: n-hexane:ethyl acetate=2:1] to obtain 9 mg of 3-amino-6-fluoro-2-pyrazinecarboxamide as a solid product. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping







