Alternatived Products of [ 34213-86-0 ]
Product Details of [ 34213-86-0 ]
| CAS No. : | 34213-86-0 |
MDL No. : | MFCD00067366 |
| Formula : |
C12H17NO6
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | MIAKOEWBCMPCQR-GCHJQGSQSA-N |
| M.W : |
271.27
|
Pubchem ID : | 122647 |
| Synonyms : |
|
Application In Synthesis of [ 34213-86-0 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 34213-86-0 ]
- 1
-
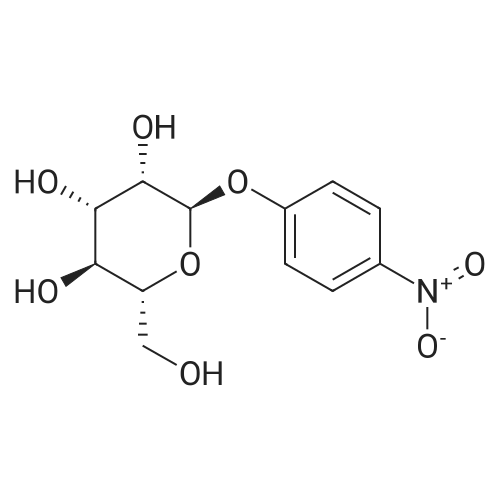 [ 10357-27-4 ]
[ 10357-27-4 ]

-
 [ 34213-86-0 ]
[ 34213-86-0 ]
| Yield | Reaction Conditions | Operation in experiment |
| 99% |
With hydrogen In water at 20℃; for 15h; atmospheric pressure; |
|
| 95% |
In methanol at 20℃; for 0.5h; |
|
| 95.49% |
With palladium on activated charcoal; hydrogen In methanol at 20℃; for 1h; |
1
Dissolve 1.07g of p-nitro D-mannose in 40mL of methanol,Add 0.05gPalladium carbon,The system was allowed to react for 1 hour in a hydrogen-filled environment.Suction filtration, the filtrate passes through a 200-300 mesh silica gel column,The mobile phase is a mixture of dichloromethane and methanol in a volume ratio of 7:1.Get p-amino D-mannose,The yield is 95.49%. |
| 95.49% |
With 5%-palladium/activated carbon In methanol for 1h; |
1
Dissolve 1.07g of p-nitro D-mannose in 40mL of methanol, add 0.05g of palladium on carbon, so that the system is filled with hydrogenReact for 1 hour in the environment. Suction filtration, the filtrate passed through a 200-300 mesh silica gel column, the mobile phase was dichloromethane and methanol by volume ratio of 7:1The prepared mixed liquid obtains p-amino D-mannose with a yield of 95.49%.[ |
| 94% |
With palladium on activated charcoal; hydrogen In methanol at 20℃; for 6h; |
|
| 85% |
With hydrogen In methanol at 20℃; for 3h; |
|
| 75% |
In diethyl ether |
1.39 p-Aminophenyl α-D-mannopyranoside
EXAMPLE 1.39 p-Aminophenyl α-D-mannopyranoside p-Nitrophenyl α-D-mannopyranoside (3.0 g, 10 mmol) is hydrogenated as described in Example 1.23. The precipitation from methanol/diethyl ether gives colourless crystals (2.03 g, 75%); TLC [methanol]: Rf=0.69; [α]20=+102.7° (c=1.0/H2O); melting point=161° C. |
| 70% |
With hydrogen In methanol at 60℃; for 4h; |
|
|
With methanol; Pd-BaSO4 Hydrogenation; |
|
|
With red. agent |
|
|
With 1% Pd/C; hydrogen In methanol; acetic acid at 20℃; for 15h; |
|
|
With hydrogen |
|

Reference:
[1]Uzawa, Hirotaka; Ito, Hiroki; Izumi, Masayuki; Tokuhisa, Hideo; Taguchi, Kazuhiro; Minoura, Norihiko
[Tetrahedron, 2005, vol. 61, # 24, p. 5895 - 5905]
[2]Nagase; Shinkai; Hamachi
[Chemical Communications, 2001, # 3, p. 229 - 230]
[3]Current Patent Assignee: HARBIN MEDICAL UNIVERSITY - CN111249234, 2020, A
Location in patent: Paragraph 0072; 0077
[4]Current Patent Assignee: HARBIN MEDICAL UNIVERSITY - CN111249235, 2020, A
Location in patent: Paragraph 0085; 0090
[5]Beiroth, Femke; Koudelka, Tomas; Overath, Thorsten; Knight, Stefan D.; Tholey, Andreas; Lindhorst, Thisbe K.
[Beilstein Journal of Organic Chemistry, 2018, vol. 14, p. 1890 - 1900]
[6]Amaike; Kobayashi; Shinkai
[Bulletin of the Chemical Society of Japan, 2000, vol. 73, # 11, p. 2553 - 2558]
[7]Current Patent Assignee: BAYER AG - US6271342, 2001, B1
[8]Downs, Frederick J.; Carroll, Robert W.
[Carbohydrate Research, 1981, vol. 88, p. 323 - 325]
[9]Westphal; Feier
[Chemische Berichte, 1956, vol. 89, p. 582,587]
[10]Kieburg, Christoffer; Lindhorst, Thisbe K.
[Tetrahedron Letters, 1997, vol. 38, # 22, p. 3885 - 3888]
[11]Hartmann, Mirja; Horst, Andrea K.; Klemm, Per; Lindhorst, Thisbe K.
[Chemical Communications, 2010, vol. 46, # 2, p. 330 - 332]
[12]Location in patent: experimental part
Grabosch, Carsten; Hartmann, Mirja; Schmidt-Lassen, Joern; Lindhorst, Thisbe K.
[ChemBioChem, 2011, vol. 12, # 7, p. 1066 - 1074]
- 2
-
 [ 50-00-0 ]
[ 50-00-0 ]

-
 [ 34213-86-0 ]
[ 34213-86-0 ]

-
 [ 74590-39-9 ]
[ 74590-39-9 ]
| Yield | Reaction Conditions | Operation in experiment |
| 85% |
With hydrogen; acetic acid In methanol for 2h; Ambient temperature; |
|
- 3
-
 [ 463-71-8 ]
[ 463-71-8 ]

-
 [ 34213-86-0 ]
[ 34213-86-0 ]

-
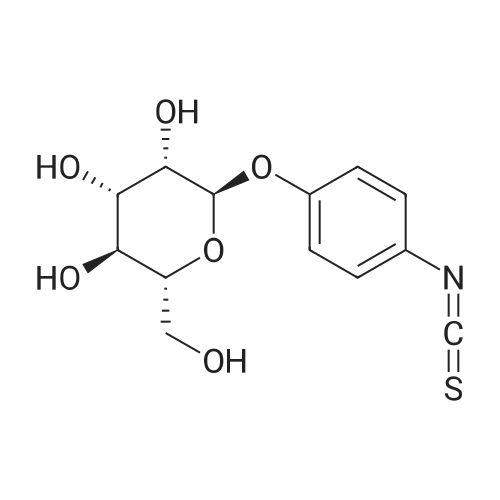 [ 96345-79-8 ]
[ 96345-79-8 ]
| Yield | Reaction Conditions | Operation in experiment |
|
In water Yield given; |
|
- 4
-
 [ 246855-91-4 ]
[ 246855-91-4 ]

-
 [ 34213-86-0 ]
[ 34213-86-0 ]

-
 [ 246855-92-5 ]
[ 246855-92-5 ]
| Yield | Reaction Conditions | Operation in experiment |
| 66% |
With diphenylphosphoranyl azide; triethylamine In N,N-dimethyl-formamide at 0 - 20℃; |
|
Reference:
[1]Li, Jun; Zacharek, Sima; Chen, Xi; Wang, Jianqiang; Zhang, Wei; Janczuk, Adam; Wang, Peng George
[Bioorganic and Medicinal Chemistry, 1999, vol. 7, # 8, p. 1549 - 1558]
- 5
-
 [ 7144-08-3 ]
[ 7144-08-3 ]

-
 [ 34213-86-0 ]
[ 34213-86-0 ]

-
3β-cholest-5-en-3-yl N-[4-(α-D-mannopyranosyl)phenyl]carbamate
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 77% |
With triethylamine In tetrahydrofuran at 60℃; for 4h; |
|
- 6
-
 [ 34213-86-0 ]
[ 34213-86-0 ]

-
p-azidophenyl-α-D-mannopyranoside
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 61% |
Stage #1: p-aminophenyl-α-D-mannopyranoside With hydrogenchloride; sodium nitrite at 0℃; for 0.5h;
Stage #2: With sodium azide at 0℃; for 0.333333h; Further stages.; |
|
- 7
-
 [ 34213-86-0 ]
[ 34213-86-0 ]

-
azobenzene-4,4'-dicarbonyl dichloride
[ No CAS ]

-
4,4'-[bis(α-D-mannopyranosyl)phenyl]azobenzene dicarboxamide
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 78% |
With triethylamine In tetrahydrofuran at 40℃; |
|
- 8
-
 [ 39093-14-6 ]
[ 39093-14-6 ]

-
 [ 34213-86-0 ]
[ 34213-86-0 ]

-
3'-[4-(α-D-mannopyranosyloxy)anilinyl]diospyrin dimethyl ether
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 86% |
In ethanol; chloroform; water at 80℃; for 34h; |
|
Reference:
[1]Sarma, Madhushree Das; Ghosh, Rina; Patra, Amarendra; Chowdhury, Rajdeep; Chaudhuri, Keya; Hazra, Banasri
[Organic and Biomolecular Chemistry, 2007, vol. 5, # 19, p. 3115 - 3125]
- 9
-
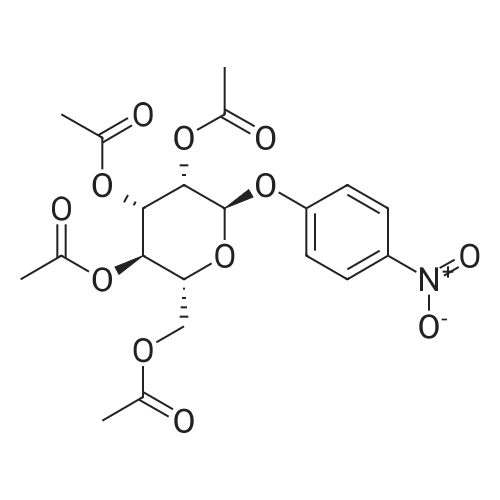 [ 13242-51-8 ]
[ 13242-51-8 ]

-
 [ 34213-86-0 ]
[ 34213-86-0 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: NaOMe / methanol / 2 h / 20 °C
2: 95 percent / Pd/C / methanol / 0.5 h / 20 °C |
|
|
Multi-step reaction with 2 steps
1: sodium methylate; methanol
2: palladium/barium sulfate; methanol / Hydrogenation |
|
|
Multi-step reaction with 2 steps
1: palladium on activated charcoal; sodium tetrahydroborate / dichloromethane; methanol / 0.25 h / 0 °C
2: sodium methylate; methanol / 16 h / 20 °C |
|
|
Multi-step reaction with 2 steps
1: sodium methylate / methanol / 20 °C
2: palladium on activated charcoal; hydrogen / methanol / 1 h / 20 °C |
|
|
Multi-step reaction with 2 steps
1: trifluoroacetic acid; sodium ethanolate / methanol
2: 5%-palladium/activated carbon / methanol / 1 h |
|

Reference:
[1]Nagase; Shinkai; Hamachi
[Chemical Communications, 2001, # 3, p. 229 - 230]
[2]Westphal; Feier
[Chemische Berichte, 1956, vol. 89, p. 582,587]
[3]Jayasundara, Dilushan R.; Duff, Thomas; Angione, M. Daniela; Bourke, Jean; Murphy, Deirdre M.; Scanlan, Eoin M.; Colavita, Paula E.
[Chemistry of Materials, 2013, vol. 25, # 20, p. 4122 - 4128]
[4]Current Patent Assignee: HARBIN MEDICAL UNIVERSITY - CN111249234, 2020, A
[5]Current Patent Assignee: HARBIN MEDICAL UNIVERSITY - CN111249235, 2020, A
- 10
-
 [ 100-02-7 ]
[ 100-02-7 ]

-
 [ 34213-86-0 ]
[ 34213-86-0 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 3 steps
1: BF3*Et2O / CH2Cl2 / 72 h / 20 °C
2: NaOMe / methanol / 2 h / 20 °C
3: 95 percent / Pd/C / methanol / 0.5 h / 20 °C |
|
|
Multi-step reaction with 3 steps
1: zinc chloride
2: sodium methylate; methanol
3: palladium/barium sulfate; methanol / Hydrogenation |
|
|
Multi-step reaction with 3 steps
1: boron trifluoride diethyl etherate / ethyl acetate / 20 °C
2: trifluoroacetic acid; sodium ethanolate / methanol
3: 5%-palladium/activated carbon / methanol / 1 h |
|
- 11
-
D-Mannose pentaacetate
[ No CAS ]

-
 [ 34213-86-0 ]
[ 34213-86-0 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 3 steps
1: BF3*Et2O / CH2Cl2 / 72 h / 20 °C
2: NaOMe / methanol / 2 h / 20 °C
3: 95 percent / Pd/C / methanol / 0.5 h / 20 °C |
|
|
Multi-step reaction with 5 steps
1: benzylamine / tetrahydrofuran / 16 h / 20 °C
2: 1,8-diazabicyclo[5.4.0]undec-7-ene / dichloromethane / 3 h / 20 °C
3: boron trifluoride diethyl etherate / dichloromethane / 2 h / -20 °C
4: palladium on activated charcoal; sodium tetrahydroborate / dichloromethane; methanol / 0.25 h / 0 °C
5: sodium methylate; methanol / 16 h / 20 °C |
|
Reference:
[1]Nagase; Shinkai; Hamachi
[Chemical Communications, 2001, # 3, p. 229 - 230]
[2]Jayasundara, Dilushan R.; Duff, Thomas; Angione, M. Daniela; Bourke, Jean; Murphy, Deirdre M.; Scanlan, Eoin M.; Colavita, Paula E.
[Chemistry of Materials, 2013, vol. 25, # 20, p. 4122 - 4128]
- 12
-
 [ 34213-86-0 ]
[ 34213-86-0 ]

-
C45H63N7O18S3
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: H2O
2: 93 percent / H2O / 12 h / Ambient temperature |
|
- 13
-
β-D-mannopyranose pentaacetate
[ No CAS ]

-
 [ 34213-86-0 ]
[ 34213-86-0 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 3 steps
1: zinc chloride
2: sodium methylate; methanol
3: palladium/barium sulfate; methanol / Hydrogenation |
|
- 14
-
 [ 98-88-4 ]
[ 98-88-4 ]

-
 [ 34213-86-0 ]
[ 34213-86-0 ]

-
 [ 186390-49-8 ]
[ 186390-49-8 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With N,N-dimethylamino-pyridine In pyridine; chloroform |
51.I I.
I. Preparation of 4-Benzamidophenyl-α-D-mannopyranoside 4-Aminophenyl-α-D-mannopyranoside (10 mg) was dissolved in pyridine and a few crystals of N,N-dimethylaminopyridine were added. The solution was cooled to 0° C. and benzoyl chloride (6 uL) was added. The mixture was stirred at 0° C. for 45 min and then at room temperature overnight. The solvent was evaporated in vacuo and chloroform (ca 1.5 mL) was added to the residue followed by enough methanol to give a clear solution. Ether was then added to the cloud point and the material was allowed to crystallize. The solvent was removed by decantation and the crystals were washed with ether. |
|
With N,N-dimethylamino-pyridine In pyridine; chloroform |
51.I I.
I. Preparation of 4-Benzamidophenyl-α-D-mannopyranoside 4-Aminophenyl-α-D-mannopyranoside (10 mg) (Sigma) was dissolved in pyridine and a few crystals of N,N-dimethylaminopyridine were added. The solution was cooled to 0° C. and benzoyl chloride (6 μl) was added. The mixture was stirred at 0° C. for 45 minutes and then at room temperature overnight. The solvent was evaporated in vacuo and chloroform (ca. 1.5 ml) was added to the residue followed by enough methanol to give a clear solution. Ether was then added to the cloud point and the material was allowed to crystallize. The solvent was removed by decanting and the crystals were washed with ether. |
- 15
-
 [ 34213-86-0 ]
[ 34213-86-0 ]

-
4-(N-phenylalanyl)-aminophenyl-α-D-mannopyranoside
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
In pyridine; methanol; dimethylsulfoxide-d6; chloroform; methoxybenzene |
51.II II.
It was then added dropwise to a cold (0° C.) solution of 4-aminophenyl-α-D-mannopyranoside in anhydrous pyridine (18 mL). The mixture was then stirred under nitrogen at 0° C. for 1 hr and at room temperature overnight. The solvent was then evaporated in vacuo. The residue was chromatographed on a flash chromatographic column using silica gel (200-400 mesh). The column was first eluted with 5% methanol in chloroform and after the non-polar impurities were eluted (TLC analysis), the eluent was changed to 10% methanol in chloroform. The fractions corresponding to the major product (TLC analysis, silica gel, 20% methanol in chloroform) were combined and evaporated to afford a white powder. The 300 MHz 1H-NMR spectrum in DMSO-d6 showed all the expected signals. The solid obtained above was dissolved in triflouroacetic acid (TFA, 10 mL) containing 4% anisole at 0° C. and the mixture was stirred at 0° C. for 90 min. The solvent was evaporated in vacuo to afford a thick oil. Cold ether was added and the mixture was allowed to stand in an ice bath for 15 min and a slid was deposited on the walls of the container. The supernatant was separated, the residue was washed 3 times with cold ether and dried overnight under high vacuum (oil pump) to afford a white solid. It was easily soluble in water. |
- 16
-
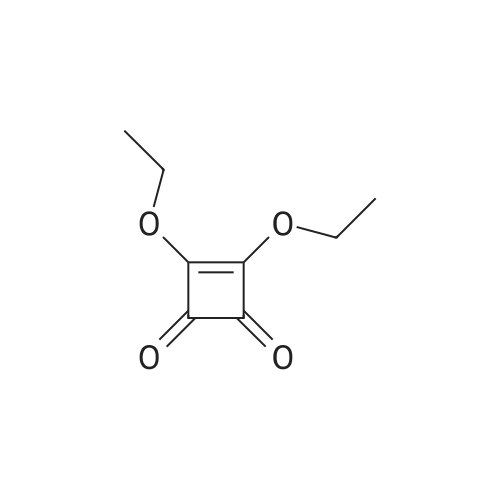 [ 5231-87-8 ]
[ 5231-87-8 ]

-
 [ 34213-86-0 ]
[ 34213-86-0 ]

-
 [ 916849-16-6 ]
[ 916849-16-6 ]
| Yield | Reaction Conditions | Operation in experiment |
| 63% |
In methanol at 20℃; for 24h; |
|
- 18
-
 [ 1425814-66-9 ]
[ 1425814-66-9 ]

-
 [ 34213-86-0 ]
[ 34213-86-0 ]

-
 [ 1425814-80-7 ]
[ 1425814-80-7 ]
| Yield | Reaction Conditions | Operation in experiment |
| 80% |
Stage #1: C20H26Cl2N2O6; p-aminophenyl-α-D-mannopyranoside With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; Inert atmosphere;
Stage #2: With silica gel In methanol; chloroform |
|
- 19
-
2,3,4,6-tetra-O-acetyl-D-mannopyranosyl trichloroacetimidate
[ No CAS ]

-
 [ 34213-86-0 ]
[ 34213-86-0 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 3 steps
1: boron trifluoride diethyl etherate / dichloromethane / 2 h / -20 °C
2: palladium on activated charcoal; sodium tetrahydroborate / dichloromethane; methanol / 0.25 h / 0 °C
3: sodium methylate; methanol / 16 h / 20 °C |
|
Reference:
[1]Jayasundara, Dilushan R.; Duff, Thomas; Angione, M. Daniela; Bourke, Jean; Murphy, Deirdre M.; Scanlan, Eoin M.; Colavita, Paula E.
[Chemistry of Materials, 2013, vol. 25, # 20, p. 4122 - 4128]
- 20
-
2,3,4,6-tetra-O-acetyl-D-mannopyranose
[ No CAS ]

-
 [ 34213-86-0 ]
[ 34213-86-0 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 4 steps
1: 1,8-diazabicyclo[5.4.0]undec-7-ene / dichloromethane / 3 h / 20 °C
2: boron trifluoride diethyl etherate / dichloromethane / 2 h / -20 °C
3: palladium on activated charcoal; sodium tetrahydroborate / dichloromethane; methanol / 0.25 h / 0 °C
4: sodium methylate; methanol / 16 h / 20 °C |
|
Reference:
[1]Jayasundara, Dilushan R.; Duff, Thomas; Angione, M. Daniela; Bourke, Jean; Murphy, Deirdre M.; Scanlan, Eoin M.; Colavita, Paula E.
[Chemistry of Materials, 2013, vol. 25, # 20, p. 4122 - 4128]
- 21
-
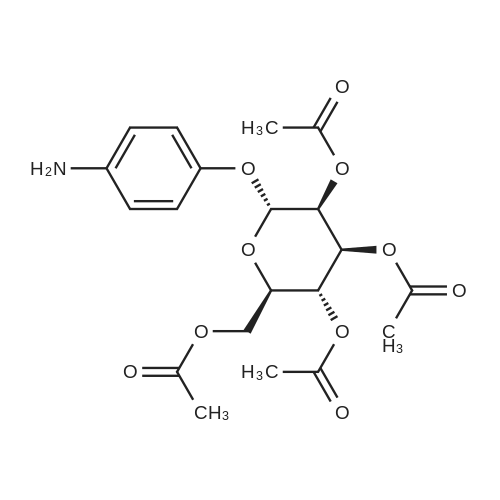 [ 187146-99-2 ]
[ 187146-99-2 ]

-
 [ 34213-86-0 ]
[ 34213-86-0 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With methanol; sodium methylate at 20℃; for 16h; |
|
Reference:
[1]Jayasundara, Dilushan R.; Duff, Thomas; Angione, M. Daniela; Bourke, Jean; Murphy, Deirdre M.; Scanlan, Eoin M.; Colavita, Paula E.
[Chemistry of Materials, 2013, vol. 25, # 20, p. 4122 - 4128]
- 22
-
bovine serum albumin
[ No CAS ]

-
 [ 34213-86-0 ]
[ 34213-86-0 ]

-
p-aminophenyl α-D-mannopyranoside complex with bovine serum albumin
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In water at 20℃; for 6.5h; |
Preparation of glycated BSA
General procedure: Man-BSA was prepared by coupling p-aminophenyl a-D-mannopyranoside to BSA through water-soluble EDC. Briefly, BSA(6.78 mg) was added to a 6-ml stirring solution of p-aminophenyl α-D-mannopyranoside (136 mg) in water (pH 4.75). Then EDC (155 mg/ml) was added dropwise over a period of 30 min at room temperature and allowed to stand for 6 h. Man-BSA was dialyzed against water and then lyophilized. Meanwhile, conjugation of carboxyl groups with p-aminophenyl β-D-glucopyranoside was also conducted for preparing glucosylated BSA (Glu-BSA). The molecularweight of Man-BSA and Glu-BSA was determined using MALDI-TOF mass analyses. |
- 23
-
 [ 75706-11-5 ]
[ 75706-11-5 ]

-
 [ 34213-86-0 ]
[ 34213-86-0 ]

-
(Z)-3-oxo-N-(4-(trifluoromethyl)phenyl)-2-((4-((2R,3S,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl) tetrahydro-2H-pyran-2-yloxy)phenylamino)methylene)butanamide
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 410 mg |
In methanol at 60℃; for 1h; stereospecific reaction; |
|
- 24
-
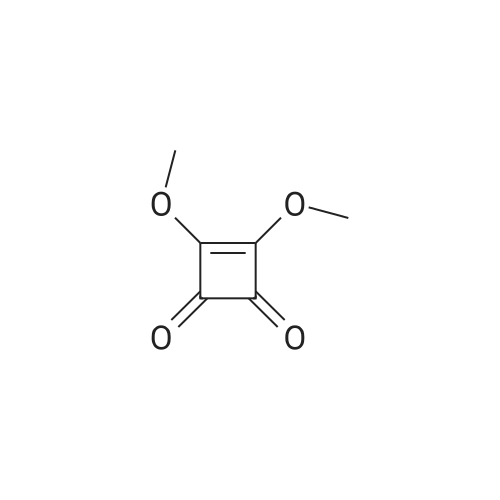 [ 5222-73-1 ]
[ 5222-73-1 ]

-
 [ 34213-86-0 ]
[ 34213-86-0 ]

-
N-[4-(α-D-mannopyranosyloxy)phenyl]amido}squaric acidmethyl ester
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 59% |
In methanol at 20℃; for 16h; |
N-[4-(α-D-Mannopyranosyloxy)phenyl]amido}squaric acidmethyl ester (10)
To a solution of the aminophenyl mannoside6 [26,27] (200 mg, 737 μmol) in dry methanol (15 mL),squaric acid dimethyl ester (9, 314 mg, 2.21 mmol) was added.The solution was stirred at room temperature for 16 h. Subsequently,the solvent was removed under reduced pressure andthe crude product was purified by flash column chromatography(ethyl acetate/methanol 3:1). The product was isolated as acolorless solid (166 mg, 436 μmol, 59%) |
- 25
-
 [ 34213-86-0 ]
[ 34213-86-0 ]

-
 [ 1417341-50-4 ]
[ 1417341-50-4 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: methanol / 16 h / 20 °C
2: triethylamine / methanol / 15 h / 20 °C |
|
- 26
-
 [ 34213-86-0 ]
[ 34213-86-0 ]

-
C14H20N2O7
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1.1: N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate / 0.75 h
1.2: 20 °C / Inert atmosphere
2.1: trifluoroacetic acid / water / 3 h / 20 °C |
|
- 27
-
 [ 34213-86-0 ]
[ 34213-86-0 ]

-
N-{2-oxo-2-[(4-(α-D-mannopyranosyloxy)phenyl)amino]-ethyl}-4-(3-trifluoromethyl-3H-diazirin-3-yl)benzamide
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 3 steps
1.1: N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate / 0.75 h
1.2: 20 °C / Inert atmosphere
2.1: trifluoroacetic acid / water / 3 h / 20 °C
3.1: N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate / 1 h
3.2: 14 h / 20 °C / Inert atmosphere |
|
- 28
-
 [ 34213-86-0 ]
[ 34213-86-0 ]

-
{N-[4-(α-D-mannopyranosyloxy)phenyl]-N’-[2’-(4’’-(3-trifluoromethyl-3H-diazirin-3-yl)phenylamido) acid diamide
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 4 steps
1.1: methanol / 16 h / 20 °C
2.1: triethylamine / methanol / 15 h / 20 °C
3.1: trifluoroacetic acid / water / 3 h / 20 °C
4.1: N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate / 0.5 h
4.2: 16 h / 20 °C / Inert atmosphere |
|
- 29
-
 [ 34213-86-0 ]
[ 34213-86-0 ]

-
 [ 1417341-51-5 ]
[ 1417341-51-5 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 3 steps
1: methanol / 16 h / 20 °C
2: triethylamine / methanol / 15 h / 20 °C
3: trifluoroacetic acid / water / 3 h / 20 °C |
|
- 30
-
 [ 34213-86-0 ]
[ 34213-86-0 ]

-
C23H25F3N2O9
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 4 steps
1.1: N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate / 0.75 h
1.2: 20 °C / Inert atmosphere
2.1: trifluoroacetic acid / water / 3 h / 20 °C
3.1: N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate / 1 h
3.2: 14 h / 20 °C / Inert atmosphere
4.1: water / dimethyl sulfoxide |
|
- 31
-
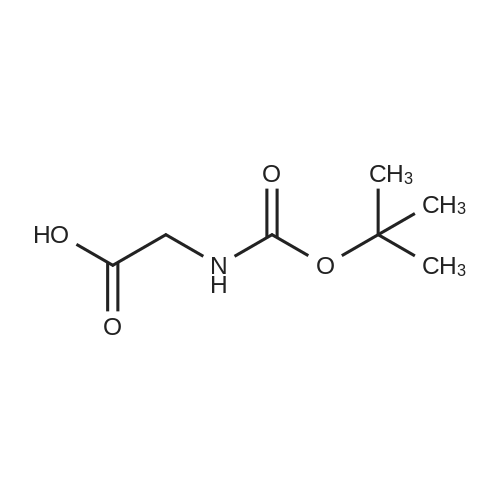 [ 4530-20-5 ]
[ 4530-20-5 ]

-
 [ 34213-86-0 ]
[ 34213-86-0 ]

-
Nα-Boc-glycin-[p-(α-D-mannopyranosyloxy)]phenyl amide
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 100% |
Stage #1: BOC-glycine; p-aminophenyl-α-D-mannopyranoside With N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate for 0.75h;
Stage #2: With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; Inert atmosphere; |
Nα-Boc-glycin-[p-(α-D-mannopyranosyloxy)]phenyl amide (7)
The aminophenyl mannoside 6 [26,27] (150 mg, 553 μmol)was dried together with N-Boc-glycine (64.6 mg, 369 μmol)and HATU (280 mg, 738 μmol) for 45 min under vacuum.Afterwards, this mixture was dissolved in dry DMF (8 mL),DIPEA (80.0 μL, 443 μmol) was added and the reaction mixturestirred overnight at room temperature. The solvent was removedunder vacuum and purified with flash column chromatography(ethyl acetate/methanol 6:1) leading to a colorless solid(158 mg, 369 μmol, quant.) |
- 32
-
C142H274N3O60P*H3N
[ No CAS ]

-
 [ 34213-86-0 ]
[ 34213-86-0 ]

-
C150H286N3O63P*H3N
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
In N,N-dimethyl-formamide at 25℃; for 24h; |
|
Reference:
[1]Li, Cong; Lai, Chaoyang; Qiu, Qiujun; Luo, Xiang; Hu, Ling; Zheng, Huangliang; Lu, Yi; Liu, Min; Zhang, Hongxia; Liu, Xinrong; Deng, Yihui; Song, Yanzhi
[AAPS PharmSciTech, 2019, vol. 20, # 5]
- 33
-
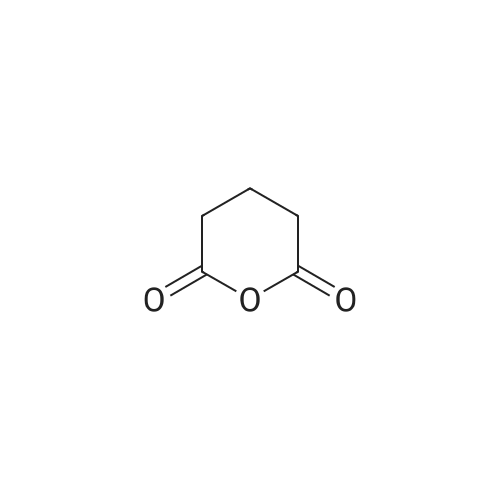 [ 108-55-4 ]
[ 108-55-4 ]

-
 [ 34213-86-0 ]
[ 34213-86-0 ]

-
C17H23NO9
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 44.3% |
With Propargylamine In dichloromethane; N,N-dimethyl-formamide for 1.5h; |
1
Dissolve 1g p-amino D-mannose in 10mL DMF (dimethylformamide), add 0.42g glutaric anhydride, at room temperatureAfter reacting for 30 minutes, add 0.24 mL propargylamine and 1.85 mL DIEA (N,N-diisopropylethylamine), and continue to react at room temperature for 1 hour. Extract with dichloromethane and water, spin-dry the water phase through a 200-300 mesh silica gel column, the mobile phase is dichloromethane and methanol by volume ratio 7:1 The mixed solution prepared to obtain para-alkynylated D-mannose, the yield is 44.3% |
|
In N,N-dimethyl-formamide at 20℃; for 0.5h; |
1
Dissolve 1g of p-amino D-mannose in 10mL DMF (dimethylformamide),Add 0.42g glutaric anhydride and react at room temperature for 30min,Then add 0.24mL propargylamine,1.85mL DIEA (N,N-diisopropylethylamine), continue to react at room temperature for 1h.Extracted with dichloromethane and water, the aqueous phase was spin-dried through a 200-300 mesh silica gel column, the mobile phase was a mixed solution of dichloromethane and methanol prepared at a volume ratio of 7:1, to obtain para-acetylated D-mannose,The yield was 44.3%. |
- 34
-
 [ 7296-15-3 ]
[ 7296-15-3 ]

-
 [ 34213-86-0 ]
[ 34213-86-0 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 4 steps
1: pyridine / 20 °C
2: boron trifluoride diethyl etherate / dichloromethane / 20 °C
3: sodium methylate / methanol / 20 °C
4: palladium on activated charcoal; hydrogen / methanol / 1 h / 20 °C |
|
- 35
-
 [ 4163-65-9 ]
[ 4163-65-9 ]

-
 [ 34213-86-0 ]
[ 34213-86-0 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 3 steps
1: boron trifluoride diethyl etherate / dichloromethane / 20 °C
2: sodium methylate / methanol / 20 °C
3: palladium on activated charcoal; hydrogen / methanol / 1 h / 20 °C |
|
|
Multi-step reaction with 3 steps
1: boron trifluoride diethyl etherate / ethyl acetate / 20 °C
2: trifluoroacetic acid; sodium ethanolate / methanol
3: 5%-palladium/activated carbon / methanol / 1 h |
|

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping

















