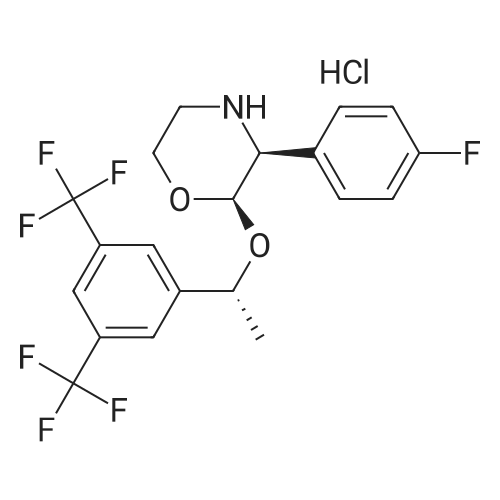
| 96% |
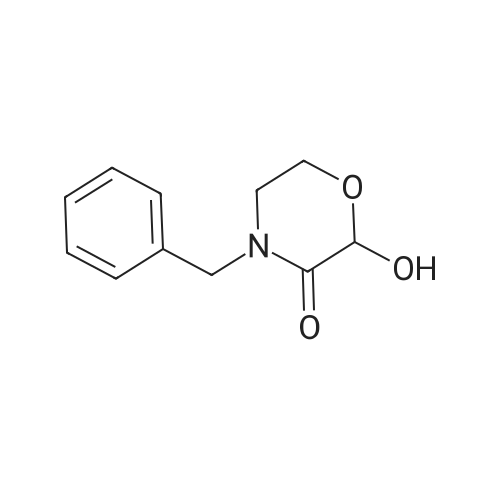
Stage #1: 4-benzyl-2-hydroxy-morpholin-3-one With trifluoroacetic anhydride In acetonitrile at 5 - 30℃; for 1h;
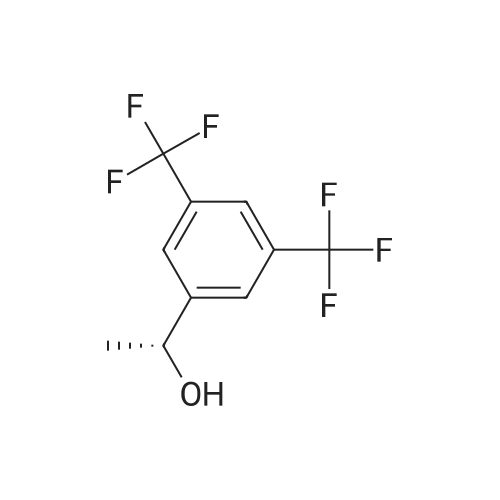
Stage #2: (R)-[3,5-bis(trifluoromethyl)phenyl]ethanol With boron trifluoride diethyl etherate In acetonitrile for 3h; Further stages; |
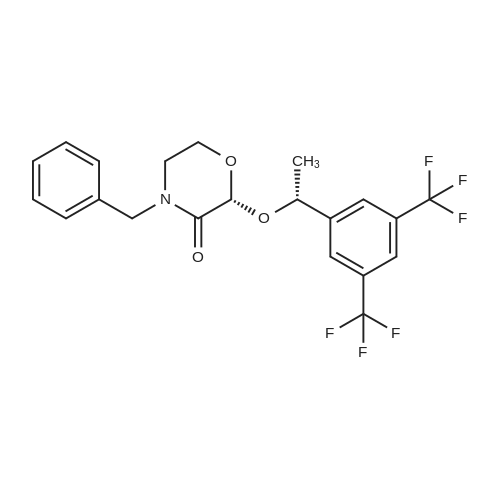
1 Example 1 Synthesis of compounds of formula II
the compound of formula VII (51.8 g, 0.250mol) is dissolved in acetonitrile (90 ml), cool at ice bath at 5 ° C, then added the drops of trifluoroacetic anhydride (52.6g, 0.250mol), the internal temperature has been raised at 30 ° C and stirring for 1 hour after that added the drops of (R)-1-[3, 5-Bis(trifluoromethyl)phenyl] ethanol (55.2g, 0.214mol) to the acetonitrile (50 ml) solution, after finished the drops again added the drops of Boron trifluoride etherate (11.5ml, 0.092mol), after stirring for 3 hours, added the drops of 5M sodium hydroxide solution (138.5ml, 0.693mol), then the acetonitrile is distilled at atmospheric pressure under the temperature of 92 ° C. Add 200ml water, extract with ethyl acetate (80 ml X 2), combined organic layer, brine (100 ml), dried, filtered, and concentrated to get dry. into the reaction flask added the potassium tert-butoxide (5.5 g, 0.09, 1.01), heptanes (300ml), and with stirring added the 3, 7-dimethyl-3'-octanol (11.0 g, 0. 069 mol), reflux for 45min after the concentration to get dry. Adding the filtrate, at -5 ~ -10 ° C added the (R, R) diastereomeric seed and stirred for 12h, filtered, and washed with n-hexane. The filtrate is added at -5~- 10 ° C for 2 h, filtered and washed with heptanes. The filter cake is added with ethyl acetate (100 ml) solution, washed sequentially with dilute acetic acid, brine, saturated with sodium bicarbonate, dried, filtered, concentrated to get dryness. Added the heptanes (150ml,) - 5 ~ -10 ° C stirring for 1h, filter, and wash with heptanes, then after drying obtained the white solid that is compound of formula II (69.8g, 96.0%). |
| 96% |
Stage #1: 4-benzyl-2-hydroxy-morpholin-3-one With trifluoroacetic anhydride In acetonitrile at 5 - 30℃; for 1h;
Stage #2: (R)-[3,5-bis(trifluoromethyl)phenyl]ethanol With boron trifluoride diethyl etherate In acetonitrile for 2h;
Stage #3: With tetrahydrolinalool; potassium <i>tert</i>-butylate In hexane at -10 - -5℃; for 14h; |
1.2 Step 2 Synthesis of the compound of formula I
The compound of the formula III (51.8 g, 0.250 mol) was dissolved in dry acetonitrile (90 ml), cooled to 5 ° C in ice water, trifluoroacetic anhydride (52.6 g, 0.250 mol) was added dropwise, and the internal temperature was raised to 30 ° C and stirred for 1 hour. Then, a solution of (R)-1-[3,5-bis(trifluoromethyl)phenyl]ethanol (55.2 g, 0.214 mol) in acetonitrile (50 ml) was added dropwise, and a solution of boron trifluoride etherate was added dropwise. (11.5 ml, 0.092 mol), after stirring for 2 hours, a 5 M sodium hydroxide solution (138.5 ml, 0.693 mol) was added dropwise, and acetonitrile was distilled at atmospheric pressure to a fraction temperature to 92 °C. After adding 200 ml of water, ethyl acetate (80 ml × 2) was combined, and the organic layer was combined, brine (50 ml × 2), dried, filtered and concentrated to dryness.Potassium tert-butoxide (5.5 g, 0.049 mol) was added to a 250 ml reaction flask.Hexane (300 ml) was added with 3,7-dimethyl-3-octanol (11.0 g, 0.069 mol) with stirring. The filtrate was added, and the (R, R) diastereomer seed crystals were added at -5 to -10 ° C for 12 h, filtered, and washed with n-hexane. The filtrate was further stirred at -5 to -10 ° C for 2 h, filtered, and washed with n-hexane. The filter cake was dissolved in ethyl acetate (100 ml), followed by dilute aqueous acetic acid (50 ml), brine (50 ml), saturated sodium bicarbonate(50 ml), brine (50 ml) washed, dried, filtered and concentrated to dry. Add n-hexane (150 ml,) at -5 to -10 ° C for 1 h, filter, wash with n-hexane and dry to give a white solid as a compound of formula I (69.8 g, 96.0%). |
|
Stage #1: 4-benzyl-2-hydroxy-morpholin-3-one With trifluoroacetic anhydride In acetonitrile at 5℃; for 1h;
Stage #2: (R)-[3,5-bis(trifluoromethyl)phenyl]ethanol With boron trifluoride diethyl etherate In acetonitrile at 25℃; for 4h; |
1
4-Benzyl-2-hydroxy-morpholin-3-oneII25g,Trifluoroacetic anhydride 50 gAnd anhydrous acetonitrile 80 gAdded to the reactor,The mixture was stirred at 5 ° C for 1 hour,Join(R) -1- [3,5-bis (trifluoromethyl) phenyl] ethanol III60g andBoron trifluoride etherate17g,The condensation reaction was stirred at 25 ° C for 4 hours,The reaction solution was treated and crystallized to give compound IV. To the reaction vessel was added magnesium 2 g,Tetrahydrofuran 40 g and p-fluorobromobenzene 16 g,The heating initiates a reaction to disappearance of magnesium,That is, the format reagent and cooling stand-by;Compound IV was dissolved in 100 g of tetrahydrofuran as a solution,The above format reagent was added dropwise until the reaction was complete,The reaction was quenched by the addition of 16 g of methanol,0.5 g of palladium on carbon was added to the reaction solution,Hydrogenation was carried out until hydrogenation was carried out until the reaction was complete,After filtration, the filtrate was evaporated to give compound . 33 g of methyl isobutyl ketone was added,3 g of sodium bicarbonate and 5 g of sodium citrate,Stirring and separating the organic layer,Organic layer by adding 37% hydrochloric acid 6g hydrochloric acidification reaction,The reaction solution was pumped into an autoclave to crystallize, centrifuged, dried,To obtain 53.1 g of the powdery product of the target product Compound I,The total yield was 93.1% (based on 4-benzyl-2-hydroxy-morpholin-3-one II) and the HPLC purity was 99.0% or more. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping