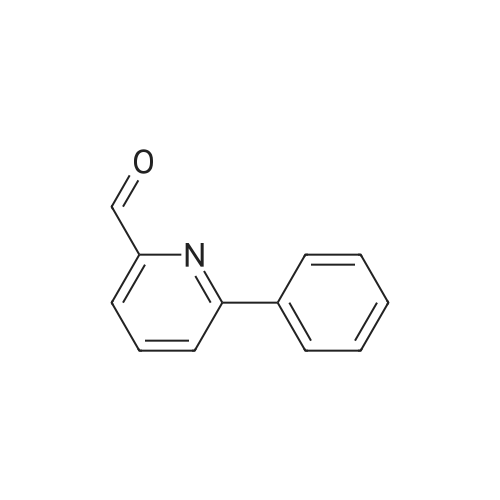
Alternatived Products of [ 238760-00-4 ]
Product Details of [ 238760-00-4 ]
| CAS No. : | 238760-00-4 |
MDL No. : | N/A |
| Formula : |
C18H20N2O
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | YVXPCSYTPPMTHS-MRXNPFEDSA-N |
| M.W : |
280.36
|
Pubchem ID : | 59767358 |
| Synonyms : |
|
Safety of [ 238760-00-4 ]
Application In Synthesis of [ 238760-00-4 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 238760-00-4 ]
- 1
-
 [ 117667-83-1 ]
[ 117667-83-1 ]

-
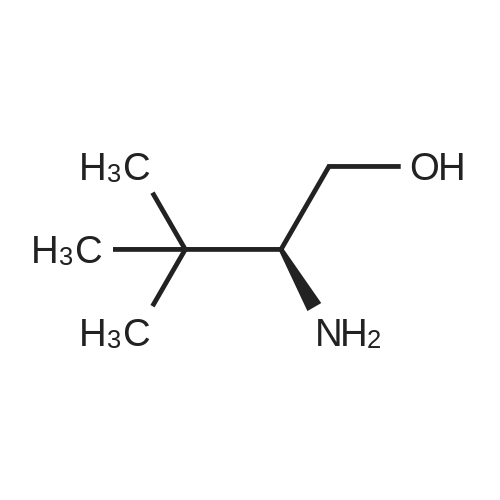 [ 112245-13-3 ]
[ 112245-13-3 ]

-
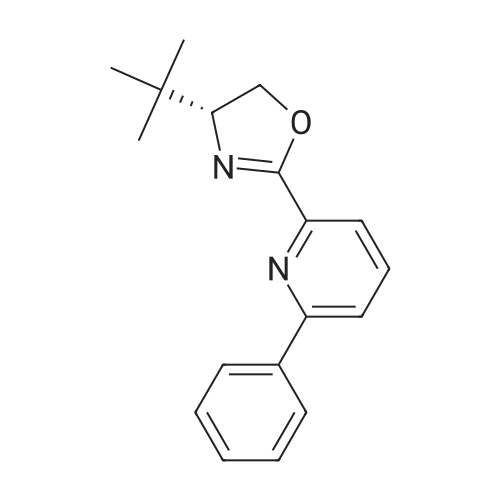
 [ 238760-00-4 ]
[ 238760-00-4 ]
| Yield | Reaction Conditions | Operation in experiment |
| 78% |
In dichloromethane at 50℃; for 16h; |
D
Methyl-6-phenylpyridyl-2-imidate (0.200 g, 0.942 mmol) and (S)-tert-leucinol (0.122 g, 1.04 mmol) were dissolved in CH2CI2 (4 ml_). The reaction vessel was sealed and heated in a heating block (50 0C) for 16 hours and the reaction mixture was then cooled to ambient temperature. The reaction mixture was loaded onto the top of a Biotage cartridge (silica gel, 40S) and the product was eluted using a stepped gradient of 0 to 40% ethyl acetate in hexanes as the eluent. The product was obtained after evaporation of the eluent as a white solid (0.205 g, 78%). |
- 2
-
 [ 98-80-6 ]
[ 98-80-6 ]

-
 [ 238760-00-4 ]
[ 238760-00-4 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1.1: tetrakis(triphenylphosphine) palladium(0)
2.1: <i>tert</i>-butyl alcohol / 2 h / 20 °C / Inert atmosphere
2.2: 16 h / 70 °C / Inert atmosphere |
|
Reference:
[1]Wang, Tao; Hao, Xin-Qi; Huang, Juan-Juan; Wang, Kai; Gong, Jun-Fang; Song, Mao-Ping
[Organometallics, 2014, vol. 33, # 1, p. 194 - 205]
- 3
-
 [ 112245-13-3 ]
[ 112245-13-3 ]

-
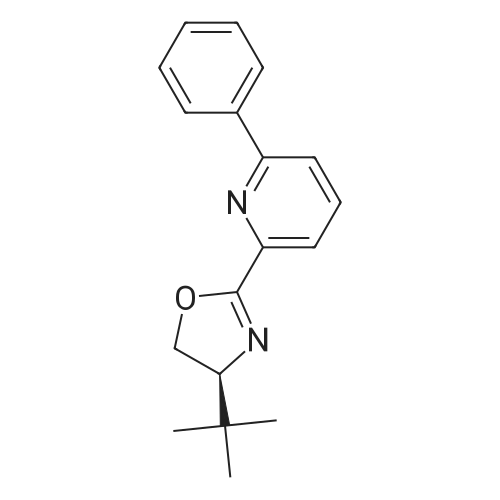
 [ 157402-44-3 ]
[ 157402-44-3 ]

-
 [ 238760-00-4 ]
[ 238760-00-4 ]
- 4
-
 [ 238760-00-4 ]
[ 238760-00-4 ]

-
palladium dichloride
[ No CAS ]

-
[(S)-4-(tert-butyl)-2-(6-phenylpyridin-2-yl)-4,5-dihydrooxazole]-palladium(II) chloride
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 48% |
With sodium hydrogencarbonate In toluene for 16h; Inert atmosphere; Reflux; |
|
Reference:
[1]Wang, Tao; Hao, Xin-Qi; Huang, Juan-Juan; Wang, Kai; Gong, Jun-Fang; Song, Mao-Ping
[Organometallics, 2014, vol. 33, # 1, p. 194 - 205]
- 5
-
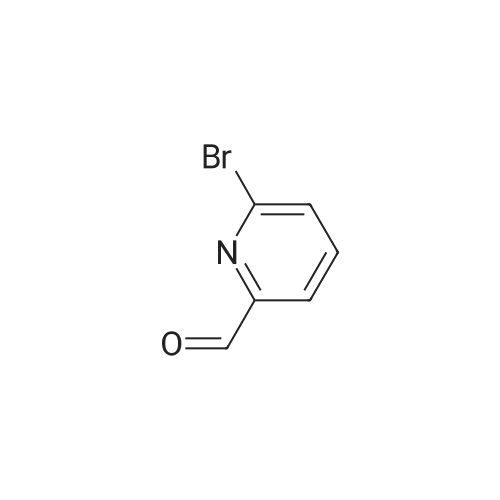 [ 34160-40-2 ]
[ 34160-40-2 ]

-
 [ 238760-00-4 ]
[ 238760-00-4 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1.1: tetrakis(triphenylphosphine) palladium(0)
2.1: <i>tert</i>-butyl alcohol / 2 h / 20 °C / Inert atmosphere
2.2: 16 h / 70 °C / Inert atmosphere |
|
Reference:
[1]Wang, Tao; Hao, Xin-Qi; Huang, Juan-Juan; Wang, Kai; Gong, Jun-Fang; Song, Mao-Ping
[Organometallics, 2014, vol. 33, # 1, p. 194 - 205]
- 6
-
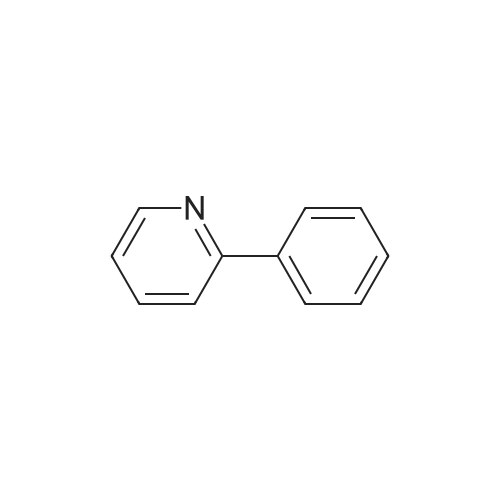 [ 1008-89-5 ]
[ 1008-89-5 ]

-
 [ 238760-00-4 ]
[ 238760-00-4 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 0 - 20 °C / Schlenk technique; Inert atmosphere
1.2: 20 °C / Schlenk technique; Inert atmosphere
2.1: sodium methoxide / methanol / 20 °C / Schlenk technique; Inert atmosphere
2.2: 80 °C / Schlenk technique; Inert atmosphere |
|
Reference:
[1]Chu, Wen-Dao; Liang, Tian-Tian; Ni, Hao; Dong, Zhi-Hong; Shao, Zhihui; Liu, Yong; He, Cheng-Yu; Bai, Ruopeng; Liu, Quan-Zhong
[Organic Letters, 2022, vol. 24, # 27, p. 4865 - 4870]
- 7
-
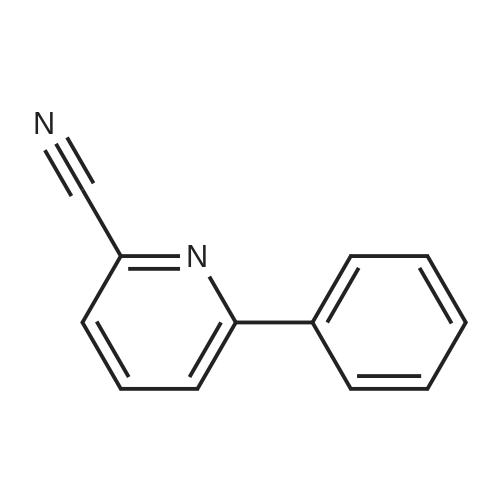 [ 39065-47-9 ]
[ 39065-47-9 ]

-
 [ 112245-13-3 ]
[ 112245-13-3 ]

-
 [ 238760-00-4 ]
[ 238760-00-4 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Stage #1: 6-phenyl-pyridine-2-carbonitrile With sodium methoxide In methanol at 20℃; Schlenk technique; Inert atmosphere;
Stage #2: L-tert-leucinol With toluene-4-sulfonic acid In toluene at 80℃; Schlenk technique; Inert atmosphere; |
|
Reference:
[1]Chu, Wen-Dao; Liang, Tian-Tian; Ni, Hao; Dong, Zhi-Hong; Shao, Zhihui; Liu, Yong; He, Cheng-Yu; Bai, Ruopeng; Liu, Quan-Zhong
[Organic Letters, 2022, vol. 24, # 27, p. 4865 - 4870]
- 8
-
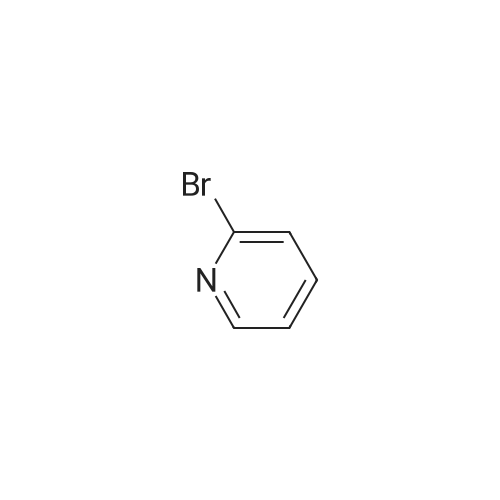 [ 109-04-6 ]
[ 109-04-6 ]

-
 [ 238760-00-4 ]
[ 238760-00-4 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 3 steps
1.1: anhydrous sodium carbonate; tetrakis-(triphenylphosphine)-palladium / ethanol; toluene; lithium hydroxide monohydrate / 4 h / Schlenk technique; Inert atmosphere; Reflux
2.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 0 - 20 °C / Schlenk technique; Inert atmosphere
2.2: 20 °C / Schlenk technique; Inert atmosphere
3.1: sodium methoxide / methanol / 20 °C / Schlenk technique; Inert atmosphere
3.2: 80 °C / Schlenk technique; Inert atmosphere |
|
Reference:
[1]Chu, Wen-Dao; Liang, Tian-Tian; Ni, Hao; Dong, Zhi-Hong; Shao, Zhihui; Liu, Yong; He, Cheng-Yu; Bai, Ruopeng; Liu, Quan-Zhong
[Organic Letters, 2022, vol. 24, # 27, p. 4865 - 4870]

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping