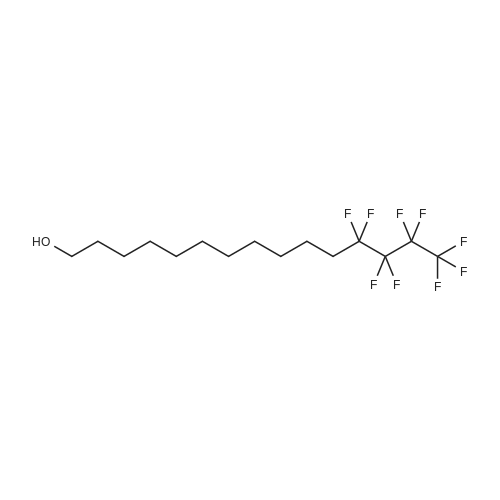
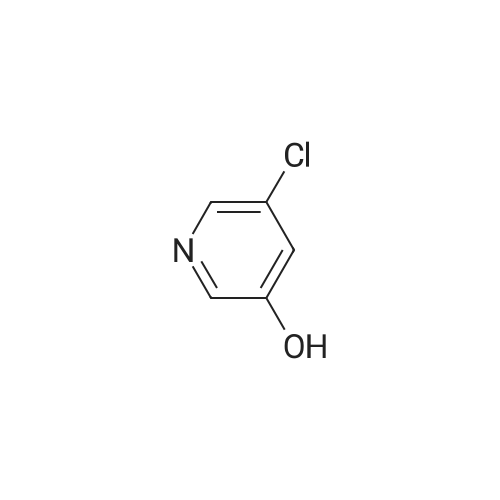
|
|
The compounds of the invention of general formula (12) can be prepared by means of stan- dard peptide chemistry (General procedure H), e. g. in 0.5 mmol scale, using Fmoc strategy and HOAt or HOBT activated amino acids. The compounds prepared in the following exam- ples according to General procedure (Q) were all isolated as the TFA salts. This procedure is further illustrated in the following: Typically, 2 gram of Fmoc Tentagel S RAM resin (Rapp Polymere, Tubingen) with substitu- tion 0,25 mmol/g was washed with NMP then treated with 25% piperidine in NMP for 30 min followed by wash with NMP which renders the resin ready for coupling. Step wise coupling OF FMOC-ARGININE (FMOC-ARG (PMC)-OH), FMOC-GLYCINE (FMOC-GLY-OH) and FMOC-4-AMINOBENZOIC ACID (FMOC-4-ABZ-OH) : To 2 mmol of Fmoc-L-Arg (Pmc) -OH (Novabiochem) was added 3,33 ml 0, 6M HOAt in NMP (Perseptives) or 0, 6M HOBT in NMP (Novabiochem) containing 0,2% BROMPHENOLBLUE as indicator and added 330 LL of diisopropylcarbodiimide DIC (Fluka) and the solution was then added to the resin. After coupling for minimum 1 hour, or when the blue colour disappeared, the resin was washed with NMP and the Fmoc group was deprotected with 25% piperidine in NMP for 20 minutes followed by wash with NMP. This stepwise assembling of the arginine residues was repeated to give 3,4, 5 or 6 arginines on the resin. The Fmoc-Glycine (No- vabiochem) and Fmoc-4-aminobenzoic acid (Fluka and Neosystems) were coupled using the same procedure as described for Fmoc-Arg (Pmc)-OH. Coupling OF A-OH, E. g. 1H-BENZOTRIAZOLE-5-CARBOXYLIC ACID on Gly. When A-OH, e. g. 1 H-BENZOTRIAZOLE-5-CARBOXYLIC acid (Aldrich) was coupled on a glycine or arginine residue the coupling procedure was as described above. Coupling of A-OH, e. g. 1H-BENZOTRIAZOLE-5-CARBOXYLIC ACID on Abz or 4-APAC : Due to the lower nucleophilicity of the amino group in Abz the following procedure was nec- essary. To 4 mmol of A-OH, e. g. 1 H-benzotriazole-5-carboxylic acid was added 6,66 ml of a solution of 0, 6M HOAt, 0,2 mmol dimethylaminopyridine (DMAP) and 4 mmol DIC and was then added to the resin and allowed to react overnight. Introduction OF FRAGMENT 4-APAC INSTEAD OF 4-ABZ : 4-Nitrophenoxyacetic acid may be coupled on a glycine or arginine residue using DIC and HOBT/HOAt as described above. Subsequent reduction of the nitro group may be done us- ing SNCI2 in NMP or DMF e. g. as described by TUMELTY et al. (Tet Lett., (1998) 7467-70). Cleavage of the peptides from the resin. After synthesis the resin was washed extensively with diethyl ether and dried. To 1 gram* of the peptidyl resin was added 25 ml of a TFA solution containing 5% thioanisole, 5% ethanol, 5% phenol and 2% triisopropylsilane and allowed to react for 2 hours. The TFA solution was filtered and concentrated with argon flow for approximately 30 minutes. Then diethylether ca. 5-7 times the residual volume of TFA was added and the peptide precipitate was extracted in 10% ACOH and washed 5 times with diethyl ether and LYOPHILIZED. RP-HPLC analysis and purification : The crude products were analysed on RP-HPLC C18 column (4,6 x 250 mm) using one of two gradients (see table 1 and 2), temperature 25C, wavelength 214 nm and flow rate 1 ml/min with A-buffer 0,15 % TFA in H20 and B- Buffer (87,5 % (W/W) MeCN, 0,13 % (w/w) TFA in H2O). The products were purified on preparative RP-HPLC C18 column (2x25 cm) using a gradient (variable, see e. g example 1013 and similar), temperature 25C, wavelength 214 nm and flow rate 6 ML/MIN with A-buffer 0, 15 % (W/W) TFA in H20 and B-Buffer (87,5 % (W/W) MECN, 0, 13 % (w/w) TFA in H2O) and verified by mass spectrometry (MALI). Table 1 : Time (min.) Flow (ml/min) %A %B ( 0 1,00 95,0 5,0 30, 00 1, 00 80, 0 20, 0 35,00 1,00 0,0 100,0 40, 00 1,00 0,0 100,0 45, 00 1, 00 95, 0 5, 0 Table 2: Time (min.) Flow (ml/min) % A % B 0 1, 00 95, 0 5, 0 30, 00 1, 00 40, 0 60, 0 31, 00 1, 00 0, 00 100, 0 35, 00 10,00 0,00 100,0 36,00 1, 00 95, 0 5, 0 The following examples were prepared using this general procedure (O). Example 1010 (General Procedure (Q)) BENZOTRIAZOL-5-YLCARBONYL-GIY2-ARG3-NH2 (BT-G2R3). MS (MALDI) : m/z: 746.7 g/mol ; calculated : 744.2 g/mol. HPLC gradient: Time (min) Flow % A % B (ml/min) 0, 00 6, 00 90, 0 10, 0 120, 00 6,00 90,0 10,0 121, 00 0,10 90,0 10,0 |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













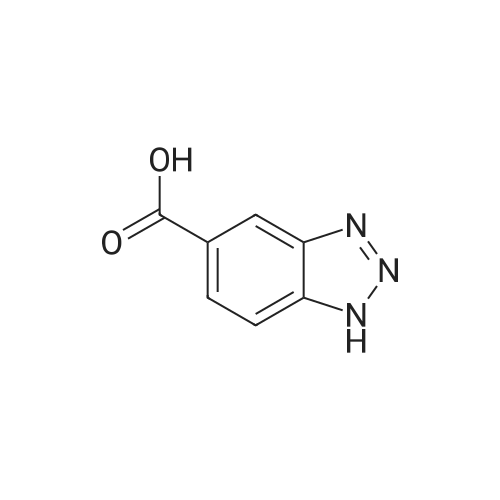

 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping