| 84% |
|
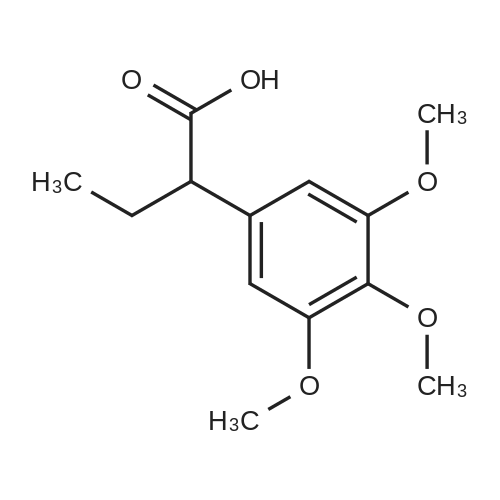
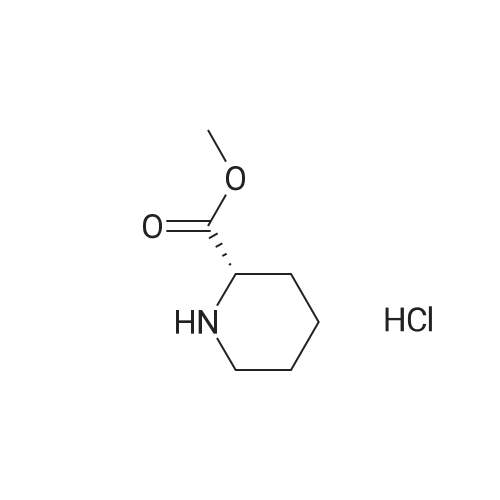
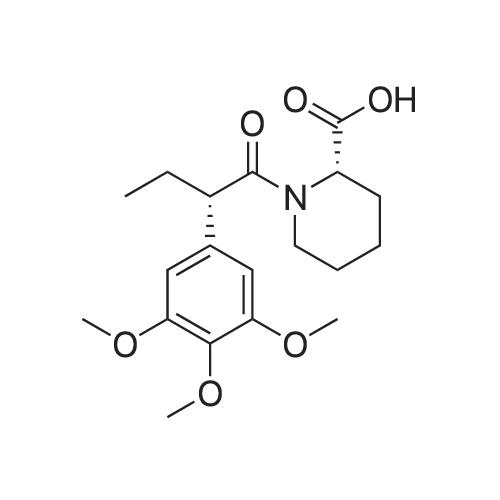
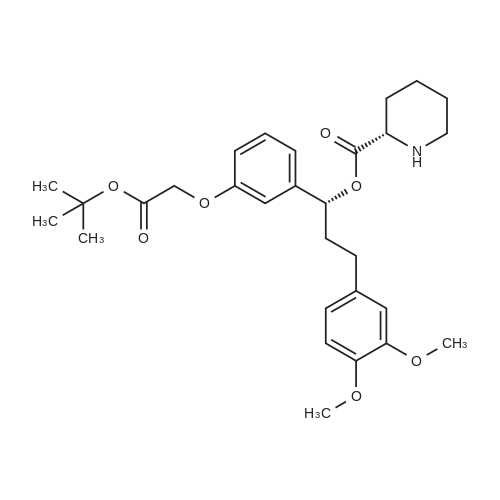
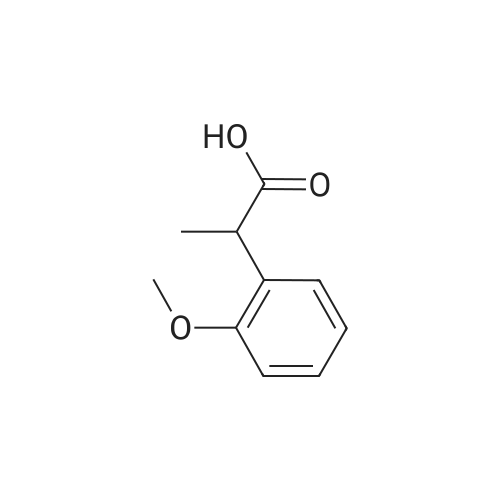
Cpd9' (99 g, 0.39 mol) was dissolved in 1 L of dichloromethane, and (S)-piperidine-2-carboxylic acid methyl ester hydrochloride (71.3 g, 0.40 mol), 2-chloro-1-methylpyridine Iodide (CMPI, 126.4 g, 0.50 mol), triethylamine (165 ml, 1.167 mol) was added dropwise, and the reaction was completed at room temperature for 2 h. TLC showed the reaction was completed and concentrated directly to give an oil. 50 ml of water, and then added LiOH (81.8 g, 3.41 mol), stirred at room temperature overnight, TLC showed the reaction was complete;Post-reaction treatment: 500 ml of water was added dropwise to the reaction, 40 C, concentrated to remove methanol, then added EA 500 ml and water 200 ml, and the extract was separated, and the organic phase was saturated NaHCO.3After washing, water was added and the aqueous phase was separated. The combined aqueous phases were acidified to pH 4 with 2N HCl solution and filtered to give white solid Cpd6' (120 g, yield 84%). |
| 73% |
|
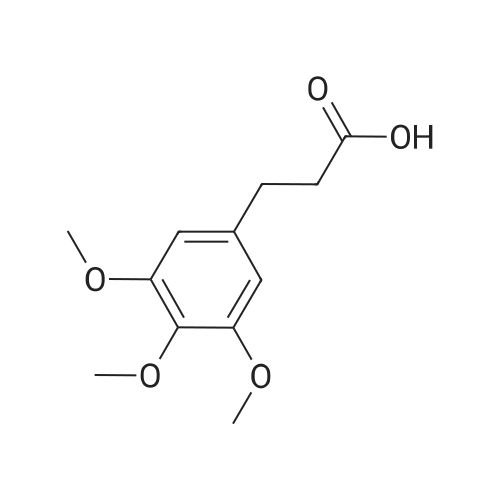
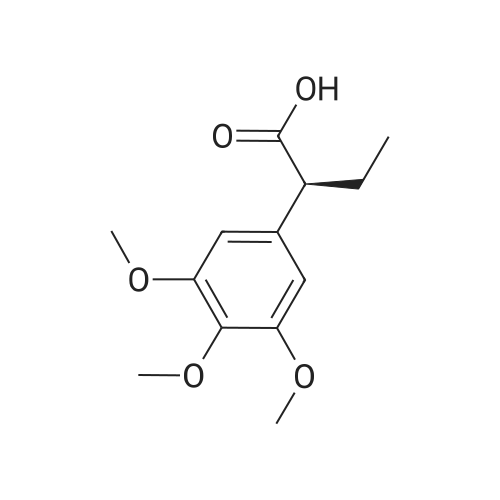
(lh) Step 8. Synthesis of [S-(R*,R*)]-l-[l-oxo-2-(3,4,5-trimethoxyphenyl)butyl]-2- piperdinecarboxylic acid (API 7362). A solution of AP17360 (10.0 g, 0.039 moles) in CH2C12 (100 mL) was treated with methyl-L- pipecolate hydrochloride (7.2 g, 0.04 moles) followed by 2-chloro-l-methylpyridinium iodide (12.77 g, 0.050 moles). The reaction mixture was then treated dropwise with triethylamine (16.66 mL, 0.120 moles) resulting in a 22C rise in the temperature of the reaction mixture. The solution was allowed to stir for 2 hours, after which time the CH2C12 was removed on a rotary evaporator, the residue (~ 50 mL volume) was dissolved in MeOH (60 mL), and the solution was treated with water (5 mL) followed by lithium hydroxide monohydrate (8.26 g, 0.197 moles). The mixture was stirred overnight then treated with water (20 mL) and the MeOH removed on a rotary evaporator at 40C. The resulting aqueous solution was treated with EtOAc (60 mL) followed by a saturated aqueous NaHC03 solution (60 mL) and the organic layer washed with water (10 mL). The combined aqueous layers were then acidified to pH 4 by careful addition of a 2N HC1 solution and the resulting suspension cooled to 10 C. The precipitate was filtered, triturated with a 1% aqueous citric acid solution (100 mL) and air dried under vacuum at 58 C to provide product, AP17362, (10.5 g, 73%): mp 173.5-174 "C; [ ] 2D +10.9 (c = 1.01, DMSO, 30 min); UV (MeOH) max 270 (epsilon 990), 232 (epsilon 11,161), 207 (epsilon 49,079) nm; NMR (DMSO- d6, 300 MHz) 6.55 (s, 2 H), 5.13 (d, J = 4.4 Hz, 1 H), 3.85-3.64 (m, 1 1 H), 2.77-2.70 (m, 1 H), 2.12 (d, J = 13.4 Hz, 1 H), 1.99-1.85 (m, 1 H), 1.65-1.55 (m, 4 H), 1.38-1.18 (m, 2 H), 0.84 (t, J = 7.2 Hz, 3 H); NMR (CD3OD, 300 MHz) 6.74 (s, 2 H), 5.43 (d, J = 4.0 Hz, 1 H), 4.13-3.83 (m, 1 1 H), 3.03 (td, J = 13.5, 3.0 Hz, 1 H), 2.44 (d, J = 13.8 Hz, 1 H), 2.24-2.14 (m, 1 H), 1.90-1.40 (m, 6 H) 1.09 (t, J = 7.3 Hz, 3 H); ,3C NMR (DMSO-d6, 75 MHz) 172.9, 172.2, 153.0, 136.2, 105.4, 60.2, 56.2, 56.0, 51.8, 49.4, 43.1, 28.5, 26.8, 25.3, 21.0, 12.8; 13C NMR (CD3OD, 75 MHz) 175.4, 174.5, 154.9, 137.5, 106.8, 61.5, 57.1, 53.9, 52.1, 45.2, 29.9, 28.2, 26.8, 22.3, 13.2; HRMS (FAB): (M-H)- calcd: 364.1760, meas: 364.1774. Anal. Calcd for C,9H2706: C, 62.45; H, 7.45; N, 3.83. Found: C, 62.32; H, 7.61 ; N, 3.88. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping