Alternatived Products of [ 192198-85-9 ]
Product Details of [ 192198-85-9 ]
| CAS No. : | 192198-85-9 |
MDL No. : | MFCD08276433 |
| Formula : |
C45H30N6
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | GEQBRULPNIVQPP-UHFFFAOYSA-N |
| M.W : |
654.76
|
Pubchem ID : | 21932919 |
| Synonyms : |
|
Safety of [ 192198-85-9 ]
Application In Synthesis of [ 192198-85-9 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 192198-85-9 ]
- 1
-
 [ 950766-71-9 ]
[ 950766-71-9 ]

-
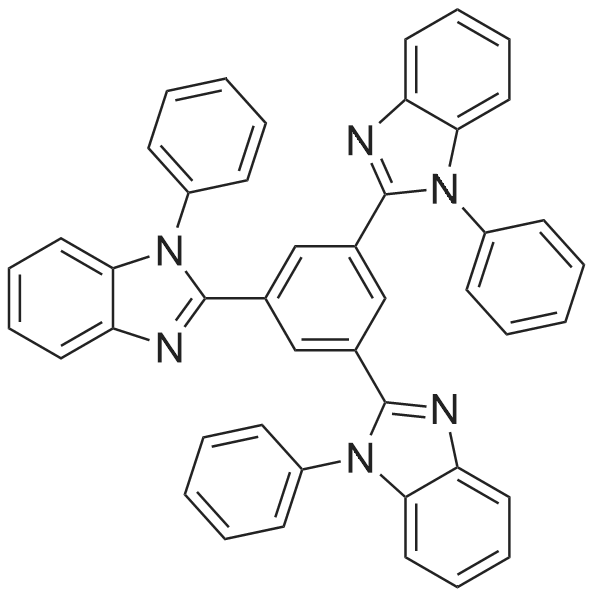 [ 192198-85-9 ]
[ 192198-85-9 ]
| Yield | Reaction Conditions | Operation in experiment |
| 70% |
With trichlorophosphate at 98℃; for 14h; Neat (no solvent); Heating / reflux; |
1
N-phenyl-1,2-phenylenediamine (0.541 mol) was combined with 1,3,5-benzene-tricarbonyl chloride (0.181 mol) in 1.8 L N-methylpyrrolidone (NMP) and stirred for 2 hrs at room temperature and then heated to 50° C. overnight. The reaction mixture was cooled to room temperature and precipitated into water (5 parts water to 1 part reaction mixture) and filtered through a medium frit then dried in a vacuum oven. Approx. 138 g of dried solid material was combined with POCl3 (0.5 kg) and carefully warmed to 98° C. for 14 hrs. The mixture was cooled to room temperature, then precipitated into stirred ice chips and water (5 parts ice and one part reaction mixture). The quenched material was neutralized with 50% NaOH to pH 9, filtered through a medium frit, and then dried in a vacuum oven. The solid was dissolved in dichloromethane (DCM), eluted through a silica plug and then purified by silica column chromatography using ethyl acetate/hexanes. 58.51 g of solid were isolated by concentrating the eluant almost to dryness, then filtering followed by drying with vacuum. The average yield for each of the two steps was 70%. |
| 400 g |
With phosphoric acid at 20 - 100℃; Industrial scale; |
1.2 Step Two
Step Two [0026]450 G of products reached by step one was mixed with 2 L of Phosphoric acid, and bronzing turbid solution reached. Then warmed up and with the increase of temperature, the redissolution of the precipitates finally finished in 2 minutes after the temperature reached 100° C. Then detected with TLC, no raw material was found. Then after cooling and poured into water, a great mass of light pink solid matters were separated out. By stoving and recrystallized by using Dichloromethane, 400 G of products reached. The hydrogen spectrums see FIG. 1. |
|
at 250℃; for 3h; |
|
Reference:
[1]Current Patent Assignee: DUPONT DE NEMOURS INC - US7273939, 2007, B1
Location in patent: Page/Page column 16
[2]Current Patent Assignee: GUANGDONG AGLAIA OPTOELECTRONIC MATERIALS CO LTD; BEIJING AGLAIA TECHNOLOGY DEVELOPMENT CO., LTD. - US2013/102788, 2013, A1
Location in patent: Paragraph 0025-0026
[3]Wang, Fangfang; Hu, Jia; Cao, Xudong; Yang, Tao; Tao, Youtian; Mei, Ling; Zhang, Xinwen; Huang, Wei
[Journal of Materials Chemistry C, 2015, vol. 3, # 21, p. 5533 - 5540]
- 2
-
 [ CAS Unavailable ]
[ CAS Unavailable ]

-
 [ CAS Unavailable ]
[ CAS Unavailable ]

-
 [ 192198-85-9 ]
[ 192198-85-9 ]
| Yield | Reaction Conditions | Operation in experiment |
|
for 24h; Reflux; |
|
Reference:
[1]Location in patent: scheme or table
Wang, Zhiming; Lu, Ping; Chen, Shuming; Gao, Zhao; Shen, Fangzhong; Zhang, Wensi; Xu, Yuanxiang; Kwok, Hoi Sing; Ma, Yuguang
[Journal of Materials Chemistry, 2011, vol. 21, # 14, p. 5451 - 5456]
- 3
-
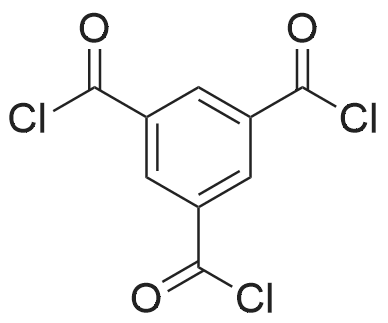 [ 4422-95-1 ]
[ 4422-95-1 ]

-
2,2',2"-(benzene-1,3,5-triyl)tris[1-phenyl-1H-benzimidazole]
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 82.5% |
With N-phenyl-1,2-benzenediamine In 1-methyl-pyrrolidin-2-one at 80 - 195℃; for 7h; |
8 Example 8 Synthesis of 1,3,5-tris(1-phenyl-1H-benzo[d]imidazol-2-yl)benzene (BZZ-13) [TPBI]
Example 8 Synthesis of 1,3,5-tris(1-phenyl-1H-benzo[d]imidazol-2-yl)benzene (BZZ-13) [TPBI] [0030] Benzene-tricarbonyl trichloride (7.4 g, 0.028 mol.) was slowly added to 50 mL of anhydrous N-Methylpyrrolidinone (NMP) containing 19.5 g (0.106 mol.) of N-phenyl-o-phenylenediamine under moisture protection. Then the reaction mixture was heated from room temperature to 80° C. under stirring for 1 hour and then raises the reaction temperature to 180° C.-195° C. for 6 hours. After the reaction mixture was cooled to room temperature, 200 mL of water was added to reaction mixture with vigorously stirring. Then resulted precipitates were filtered and washed with water, mixture of water/alcohol. The pure product of 1,3,5-tris(1-phenyl-1H-benzo[d]imidazol-2-yl)benzene (BZZ-13) [TPBI] (15.1 g) was obtained at yield of 82.5% by finally washed with acetone and dried. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping






