|
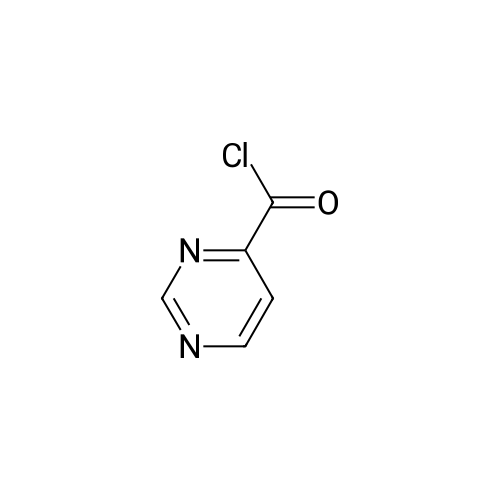
With oxalyl dichloride; N,N-dimethyl-formamide; In dichloromethane; at 0 - 27℃; for 2.16667h; |
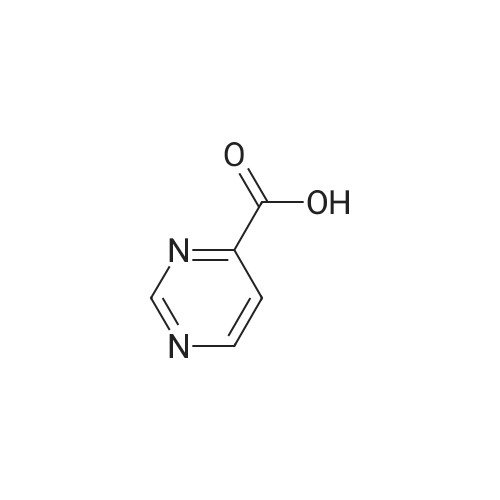
General procedure: Typical procedure for the preparation of intermediates via pyrimidine ring formation from heteroaryl carboxylic acids as exemplified by the preparation of Intermediate 8, 6-chloro-4,4'-bipyrimidine4-Pyrimidinecarboxylic acid (Intermediate 9, 3.0 g, 24.2 mmol) in DMF (0.01 mL) and CH2CI2(60 mL) was cooled to 0 C before the dropwise addition over 10 min of oxalyl chloride (2.7 mL, 31.5 mmol). After stirring at rt for 2 h the reaction mixture was concentrated in vacuo and the resulting crude acid chloride redissolved in THF (10 mL). Separately, EtOAc (8.3 mL, 84.6 mmol) was dissolved in THF (30 mL) and cooled to -78 C before the dropwise addition of LDA (36.0 mL of a 2 M solution in THF, 72.0 mmol). After stirring at -78 C for 1 h the THF solution of the crude acid chloride was added and the mixture stirred at -78 C for 3 h. Aqueous HCl (IN, 25 mL) was added, followed by H20 (100 mL) and EtOAc (100 mL), and the phases were separated. The aqueous phase was extracted with EtOAc (3 x 100 mL) and the combined organic phases were dried (Na2S04) and concentrated in vacuo. Purification by gradient flash chromatography, eluting with 0-8% EtOAc in hexane yielded ethyl 3-oxo-3-(pyrimidin-4-yl)propanoate (0.40 g, 2.06 mmol) as a white solid.TLC: Rf 0.6, Hexane / Ethyl acetate 4: 1Ethyl 3-oxo-3-(pyrimidin-4-yl)propanoate (1.2 g, 6.18 mmol), sodium methoxide (1.33 g, 24.6 mmol) and formamidine hydrochloride (1.0 g, 12.4 mmol) were dissolved in MeOH (20 mL) and stirred at room temperature for 24 h before concentration in vacuo. H20 (50 mL) and EtOAc (50 mL) were added and the phases were separated. The aqueous phase was extracted with EtOAc (3 x 50 mL) and the combined organic phases were dried (Na2S04), and concentrated in vacuo to yield crude [4,4'-bipyrimidin]-6-ol (200 mg) which was used in the next step without further purification.TLC: Rf 0.1, Ethyl acetateCrude [4,4'-Bipyrimidin]-6-ol (120 mg, 0.69 mmol) was dissolved in phosphorus(V) oxychloride (4.0 mL, 42.9 mmol) and stirred at rt for 14 h. The mixture was neutralized to approximately pH 7 with saturated aqueous NaHC03solution (30 mL) at 0 C and stirred for 15 min before the addition of EtOAc (300 mL) and H20 (100 mL). The phases were separated, the aqueous phase was extracted with EtOAc (300 mL), and the combined organic phases were dried (Na2S04) and concentrated in vacuo. Purification by gradient flash chromatography, eluting with 0-9% EtOAc in hexane yielded the title compound (42 mg, 0.22 mmol) as a white solid.Data in table 1. |
|
With oxalyl dichloride; N,N-dimethyl-formamide; In dichloromethane; at 0 - 20℃; for 2h;Inert atmosphere; |
General procedure: 2-(Benzyloxy)-3,5-dibromobenzamide (1.0 g, 2.58 mmol) was dissolved in BH3*THF (13.24 mL, 13.24 mmol) under N2 and the mixture was heated to reflux. The reaction stirred for 48 h at 75 C and was monitored by TLC (10 mL additional BH3*THF was added at 30 h). Methanol (10 mL*2) was added dropwise to quench the reaction, and then the solvent was removed in vacuo. The product was used crude in the subsequent step without purification. 1H NMR (400 MHz, DMSO-d6) delta 7.95 (d, J = 2.5 Hz, 1H), 7.91 (s, 1H), 7.74 (s, 1H), 7.60 (d, J = 2.4 Hz, 1H), 7.48-7.42 (m, 2H), 7.40-7.29 (m, 3H), 4.95 (s, 2H), 4.36 (t, 2H). 13C NMR (101 MHz, DMSO-d6) delta 151.79, 136.32, 136.20, 134.68, 131.19, 128.40, 128.39, 119.03, 116.67, 60.79, 29.25. 2-Nitrobenzonic acid (534.8 mg, 3.20 mmol) was dissolved in CH2Cl2 and the solution was cooled to 0 C under N2. Oxalyl chloride (0.550 mL, 6.40 mmol) was added dropwise, followed by DMF (1 drop). The reaction was allowed to warm to room temperature as it stirred for 2 h. Solvent and excess oxalyl chloride was removed in vacuo and then the residue was resuspended in CH2Cl2. This acid chloride was added dropwise to a solution of (2-(benzyloxy)-3,5-dibromophenyl)methanamine (655 mg, 1.60 mmol) in CH2Cl2 (32 mL, 0.1 M). Triethylamine (1.56 mL, 11.2 mmol) was added, followed by DMAP (cat.) and the mixture stirred overnight at room temperature. After quenching the reaction with water (25 mL), more CH2Cl2 was added (50 mL) and the organic layer was washed with HCl (50 mL, 1 M), sat. NaHCO3 (50 mL) and then brine (50 mL), and then was dried over Na2SO4. Purification via silica-gel column chromatography (product elutes around 30% EtOAc) afforded 354 mg of the desired product, 42.5% yield. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping