Alternatived Products of [ 168427-74-5 ]
Product Details of [ 168427-74-5 ]
| CAS No. : | 168427-74-5 |
MDL No. : | MFCD02682961 |
| Formula : |
C13H19N5O5
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | PUDXUJRJLRLJIU-QYVSTXNMSA-N |
| M.W : |
325.32
|
Pubchem ID : | 11393191 |
| Synonyms : |
|
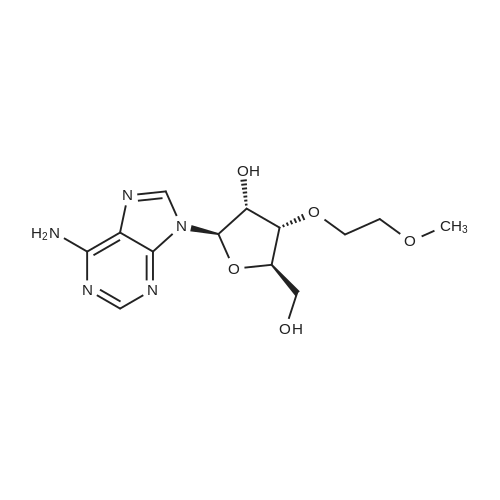
Chemical Name : | (2R,3R,4R,5R)-5-(6-Amino-9H-purin-9-yl)-2-(hydroxymethyl)-4-(2-methoxyethoxy)tetrahydrofuran-3-ol |
Safety of [ 168427-74-5 ]
Application In Synthesis of [ 168427-74-5 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 168427-74-5 ]
- 1
-
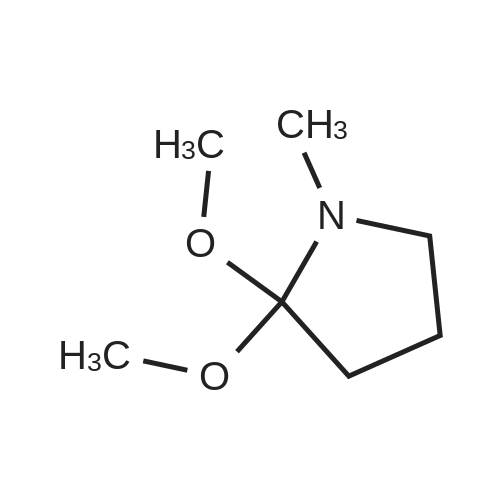 [ 39650-82-3 ]
[ 39650-82-3 ]

-
 [ 168427-74-5 ]
[ 168427-74-5 ]

-
(2R,3R,4R,5R)-2-Hydroxymethyl-4-(2-methoxy-ethoxy)-5-{6-[1-methyl-pyrrolidin-(2E)-ylideneamino]-purin-9-yl}-tetrahydro-furan-3-ol
[ No CAS ]
- 2
-
 [ 168427-73-4 ]
[ 168427-73-4 ]

-
 [ 168427-74-5 ]
[ 168427-74-5 ]
- 3
-
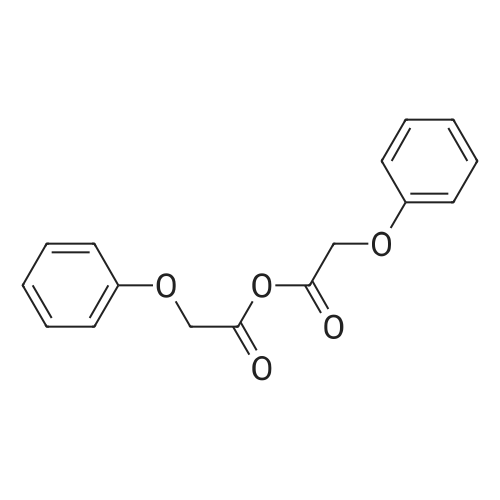 [ 14316-61-1 ]
[ 14316-61-1 ]

-
 [ 168427-74-5 ]
[ 168427-74-5 ]

-
N-{9-[(2R,3R,4R,5R)-4-Hydroxy-5-hydroxymethyl-3-(2-methoxy-ethoxy)-tetrahydro-furan-2-yl]-9H-purin-6-yl}-2-phenoxy-acetamide
[ No CAS ]
- 4
-
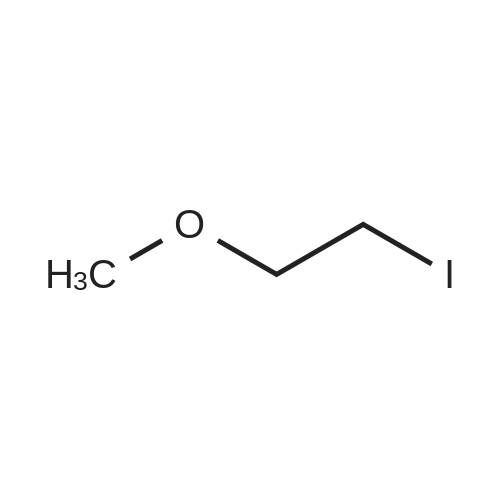 [ 4296-15-5 ]
[ 4296-15-5 ]

-
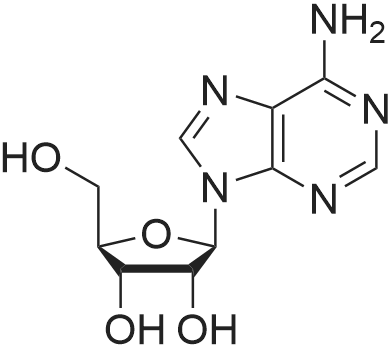 [ 58-61-7 ]
[ 58-61-7 ]

-
 [ 168427-74-5 ]
[ 168427-74-5 ]
- 5
-
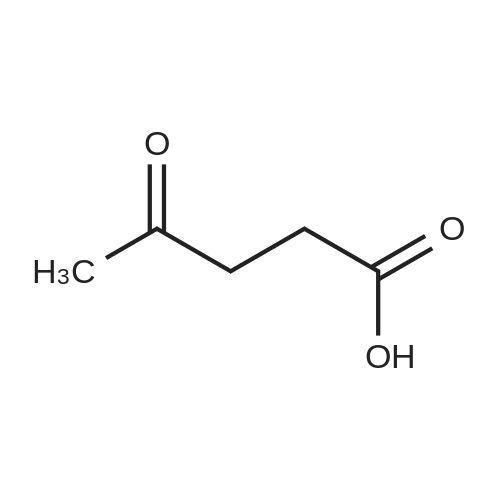 [ 123-76-2 ]
[ 123-76-2 ]

-
 [ 168427-74-5 ]
[ 168427-74-5 ]

-
 [ 440327-54-8 ]
[ 440327-54-8 ]
- 6
-
 [ 440327-54-8 ]
[ 440327-54-8 ]

-
 [ 168427-74-5 ]
[ 168427-74-5 ]

-
5'-O-levulinyl-2'-O-(2-methoxyethyl)adenosine
[ No CAS ]

-
3'-O-levulinyl-2'-O-(2-methoxyethyl)adenosine
[ No CAS ]
- 7
-
 [ 440327-54-8 ]
[ 440327-54-8 ]

-
 [ 168427-74-5 ]
[ 168427-74-5 ]

-
3'-O-levulinyl-2'-O-(2-methoxyethyl)adenosine
[ No CAS ]
- 8
-
 [ 168427-74-5 ]
[ 168427-74-5 ]

-
 [ 647834-80-8 ]
[ 647834-80-8 ]

-
5'-O-levulinyl-2'-O-(2-methoxyethyl)adenosine
[ No CAS ]
- 9
-
 [ 168427-74-5 ]
[ 168427-74-5 ]

-
5'-O-levulinyl-2'-O-(2-methoxyethyl)adenosine
[ No CAS ]
- 10
-
 [ 168427-74-5 ]
[ 168427-74-5 ]

-
3'-O-levulinyl-2'-O-(2-methoxyethyl)adenosine
[ No CAS ]
- 11
-
 [ 168427-74-5 ]
[ 168427-74-5 ]

-
N-{9-[(2R,3R,4R,5R)-5-[Bis-(4-methoxy-phenyl)-phenyl-methoxymethyl]-4-hydroxy-3-(2-methoxy-ethoxy)-tetrahydro-furan-2-yl]-9H-purin-6-yl}-2-phenoxy-acetamide
[ No CAS ]
- 12
-
 [ 168427-74-5 ]
[ 168427-74-5 ]

-
Diisopropyl-phosphoramidous acid (2R,3R,4R,5R)-2-[bis-(4-methoxy-phenyl)-phenyl-methoxymethyl]-4-(2-methoxy-ethoxy)-5-[6-(2-phenoxy-acetylamino)-purin-9-yl]-tetrahydro-furan-3-yl ester 2-cyano-ethyl ester
[ No CAS ]
- 13
-
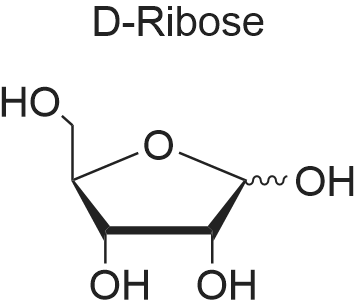 [ 50-69-1 ]
[ 50-69-1 ]

-
D-<3>pentulose
[ No CAS ]

-
 [ 168427-74-5 ]
[ 168427-74-5 ]
- 14
-
 [ 168427-74-5 ]
[ 168427-74-5 ]

-
(2R,3R,4R,5R)-2-[Bis-(4-methoxy-phenyl)-phenyl-methoxymethyl]-4-(2-methoxy-ethoxy)-5-{6-[1-methyl-pyrrolidin-(2E)-ylideneamino]-purin-9-yl}-tetrahydro-furan-3-ol
[ No CAS ]
- 15
-
 [ 168427-74-5 ]
[ 168427-74-5 ]

-
5'-O-(4,4'-Dimethoxytrityl)-2'-O-(2-methoxyethyl)-N6-(1-methylpyrrolidin-2-yliden)adenosin-3'-<(2-cyanoethyl)-N,N-diisopropylphosphoramidit>
[ No CAS ]
- 16
-
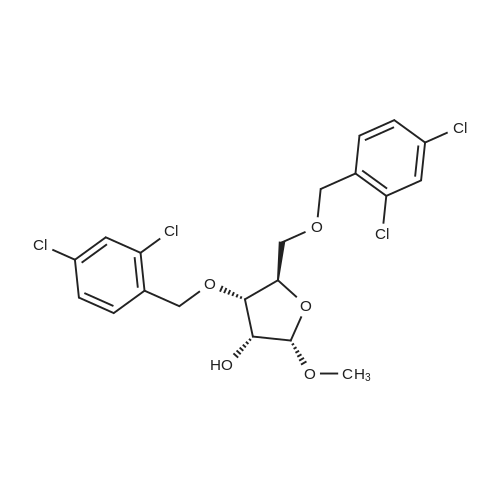 [ 168427-35-8 ]
[ 168427-35-8 ]

-
 [ 168427-74-5 ]
[ 168427-74-5 ]
- 17
-
 [ 163759-42-0 ]
[ 163759-42-0 ]

-
 [ 168427-74-5 ]
[ 168427-74-5 ]
- 18
-
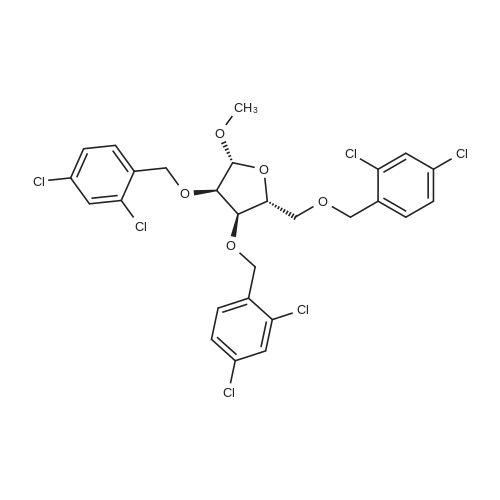 [ 163759-40-8 ]
[ 163759-40-8 ]

-
 [ 168427-74-5 ]
[ 168427-74-5 ]
- 19
-
 [ 168427-71-2 ]
[ 168427-71-2 ]

-
 [ 168427-74-5 ]
[ 168427-74-5 ]
- 20
-
 [ 168427-70-1 ]
[ 168427-70-1 ]

-
 [ 168427-74-5 ]
[ 168427-74-5 ]
- 21
-
 [ 168427-69-8 ]
[ 168427-69-8 ]

-
 [ 168427-74-5 ]
[ 168427-74-5 ]
- 22
-
 [ 168427-72-3 ]
[ 168427-72-3 ]

-
 [ 168427-74-5 ]
[ 168427-74-5 ]
- 23
-
ammonium 2'-(O-2-methoxyethyl)-adenosine-5'-monophosphate
[ No CAS ]

-
 [ 168427-74-5 ]
[ 168427-74-5 ]
- 24
-
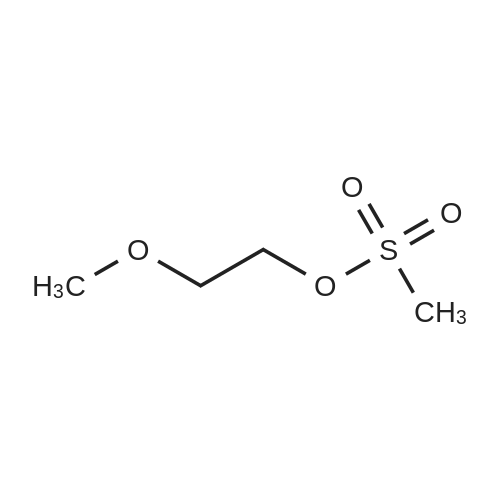 [ 16427-44-4 ]
[ 16427-44-4 ]

-
 [ 58-61-7 ]
[ 58-61-7 ]

-
 [ 303197-30-0 ]
[ 303197-30-0 ]

-
 [ 1062491-29-5 ]
[ 1062491-29-5 ]

-
 [ 1062491-27-3 ]
[ 1062491-27-3 ]

-
 [ 168427-74-5 ]
[ 168427-74-5 ]
- 25
-
 [ 16427-44-4 ]
[ 16427-44-4 ]

-
 [ 58-61-7 ]
[ 58-61-7 ]

-
 [ 303197-30-0 ]
[ 303197-30-0 ]

-
 [ 1062491-29-5 ]
[ 1062491-29-5 ]

-
 [ 168427-74-5 ]
[ 168427-74-5 ]
- 26
-
 [ 16427-44-4 ]
[ 16427-44-4 ]

-
 [ 58-61-7 ]
[ 58-61-7 ]

-
 [ 168427-74-5 ]
[ 168427-74-5 ]
| Yield | Reaction Conditions | Operation in experiment |
| 56% |
With 2-methoxy-ethanol; sodium hydroxide; In N,N-dimethyl-formamide; at 8 - 65℃;Large scale; |
10kg of Adenosine was added to 130L of anhydrous DMF and stirred to dissolve, then 50L anhydrous ethylene glycol monomethyl ether was added controlling the temperature 8-15C , added 6.89Kg of <strong>[16427-44-4]2-methoxyethyl methanesulfonate</strong> and 2.99Kg solid sodium hydroxide, stirred reaction was incubated 60-65C , HPLC monitoring completion of the reaction to the main raw material, then concentrated under reduced pressure at 60 degrees to dryness to give a brown oil as 2'-O-(2-methoxyethyl) adenosine. |
- 27
-
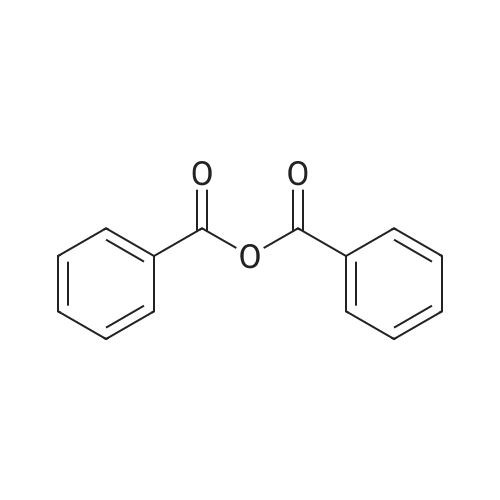 [ 93-97-0 ]
[ 93-97-0 ]

-
 [ 168427-74-5 ]
[ 168427-74-5 ]

-
N<SUP>6</SUP>-Bz-2'-O-(2-methoxyethyl)adenosine
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
With triethylamine; In acetonitrile; at 8 - 25℃;Large scale; |
5kg of 2'-O-(2-methoxyethyl) adenosine was added to a 65L anhydrous acetonitrile, with stirring, then triethylamine was added 5L controlled temperature 8-15C, 4.17Kg added benzoic anhydride, was incubated the reaction was stirred for 20-25C, the HPLC to monitor the completion of the reaction the main raw material, and then concentrated to dryness under reduced pressure at 45C to give a light yellow oil as N6-benzyl-<strong>[168427-74-5]2'-O-(2-methoxyethyl)adenosine</strong> |
- 28
-
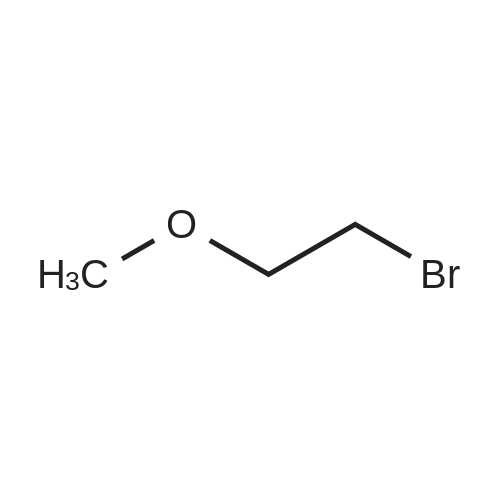 [ 6482-24-2 ]
[ 6482-24-2 ]

-
 [ 58-61-7 ]
[ 58-61-7 ]

-
 [ 168427-74-5 ]
[ 168427-74-5 ]
| Yield | Reaction Conditions | Operation in experiment |
| 20.3% |
With sodium hydride; In N,N-dimethyl-formamide; mineral oil; at 20℃; for 9h;Cooling with ice; Inert atmosphere; |
Adenosine (7.0 g, 1 eq) was dissolved in 200 mL of anhydrous DMF, NaH (1.6 g, 1.5 eq), purity 60%, was added under ice-cooling with nitrogen and stirred well for 30 min.A solution of 2-bromoethyl methyl ether (2.9 mL, 1.2 eq) was added dropwise,After 30 min, the ice bath was removed and stirred at room temperature for 8 h.After the reaction, ice water (5 mL) was added and stirred for 10 min. The reaction solution was concentrated,O-MOE-adenosine (1.7 g, yield 20.3%) was isolated by silica gel column chromatography. |
- 29
-
 [ 168427-74-5 ]
[ 168427-74-5 ]

-
5'-O-(4,4'-dimethoxytriphenylmethyl)-2'-O-(2-methoxyethyl)-N6-benzoyladenosine
[ No CAS ]
- 30
-
 [ 98-88-4 ]
[ 98-88-4 ]

-
 [ 168427-74-5 ]
[ 168427-74-5 ]

-
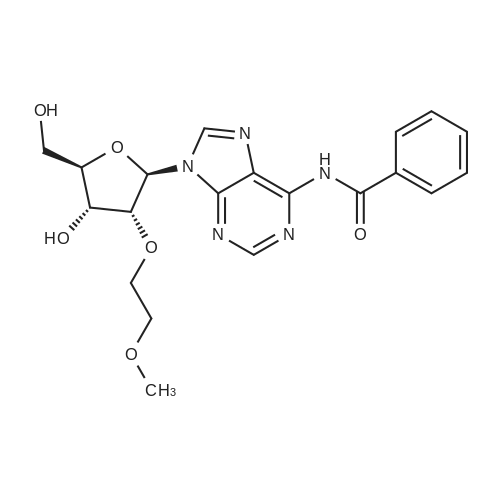 [ 333335-93-6 ]
[ 333335-93-6 ]
| Yield | Reaction Conditions | Operation in experiment |
| 87.1% |
|
The compound <strong>[168427-74-5]2'-O-MOE-adenosine</strong> (2.0 g, 1 eq) was taken, Using anhydrous pyridine 7mL With water twice, Add 10mL anhydrous pyridine to dissolve, The mixture was stirred in an ice bath for 20 min under nitrogen. Trimethylchlorosilane (4.7 mL, 8 eq) was added, After stirring for 60 min, benzoyl chloride (1.1 mL, 1.5 eq) was added, Stirring was continued for 120 min, After removing the ice bath and stirring for 30 min at room temperature, Add ice water 3mL, Stirring 30min then concentrated ammonia 5mL room temperature stirring 30min. The reaction mixture was concentrated and partitioned between methylene chloride and saturated brine, The dichloromethane phase was dried over anhydrous sodium sulfate overnight, Concentrated, and separated by column chromatography to give 2.3 g of a product in a yield of 87.1% |
- 31
-
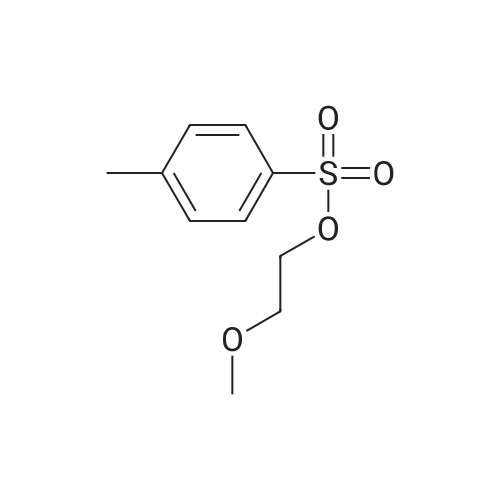 [ 17178-10-8 ]
[ 17178-10-8 ]

-
 [ 58-61-7 ]
[ 58-61-7 ]

-
 [ 168427-74-5 ]
[ 168427-74-5 ]
- 32
-
 [ 168427-74-5 ]
[ 168427-74-5 ]

-
C52H53N6O10P
[ No CAS ]
- 33
-
 [ 168427-74-5 ]
[ 168427-74-5 ]

-
C52H53N6O10P
[ No CAS ]

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping




















