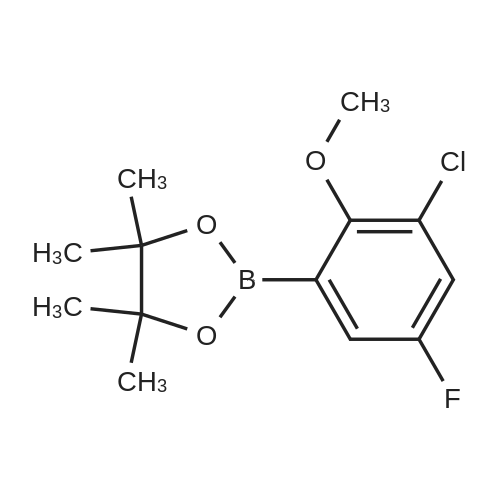
|
With (1,5-cyclooctadiene)(methoxy)iridium(I) dimer; 4,4'-di-tert-butyl-2,2'-bipyridine In tetrahydrofuran at 80℃; for 12h; Inert atmosphere; Glovebox; Sealed tube; Overall yield = 89 %; Overall yield = 53.5 mg; regioselective reaction; |
1 Example 1
General Procedure for the Borylation of 1-Chloro-3-fluoro-2-Substituted Benzenes
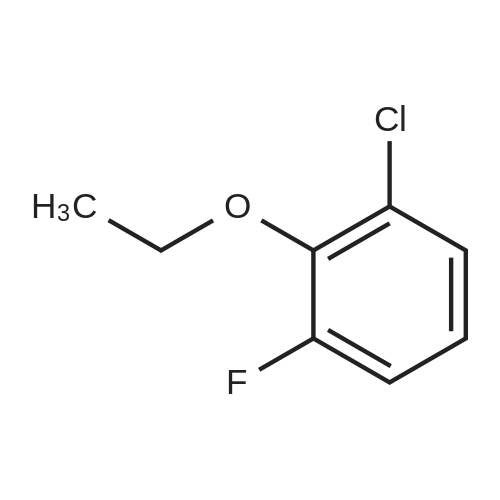
Borylation of 1-chloro-2-ethoxy-3-fluorobenzene
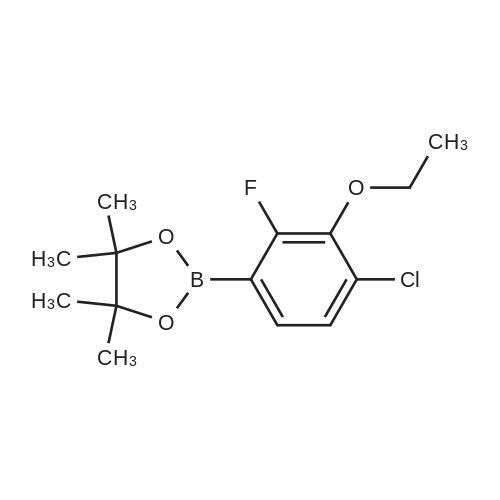
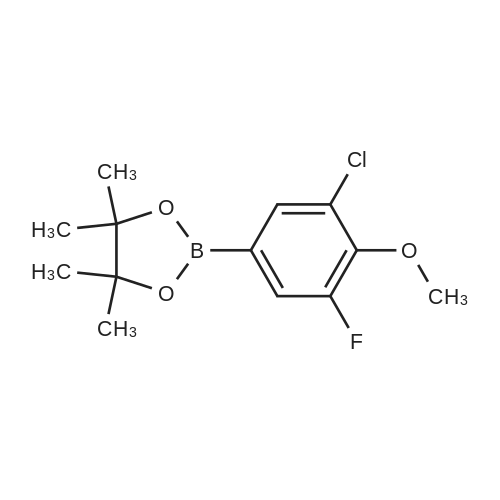
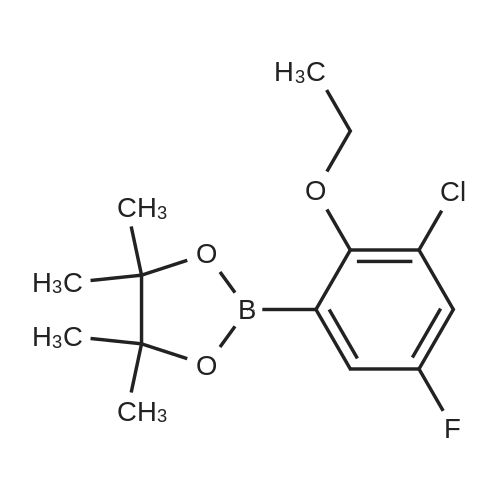
General procedure: Example 1General Procedure for the Borylation of 1-Chloro-3-fluoro-2-Substituted BenzenesBorylation of 1-chloro-2-ethoxy-3-fluorobenzeneIn a nitrogen atmosphere glovebox, bis(pinacolato)diboron (B2Pin2; 254 milligrams (mg), 1.0 millimole (mmol)) was weighed into a 20 milliliter (mL) vial and dissolved in THF (2 mL). The resulting solution was transferred to a 10 mL volumetric flask. The vial was washed with THF (3×1 mL), and the resulting solutions were transferred to the volumetric flask. The solution in the volumetric flask was diluted to the 10 mL mark with THF, giving a 0.10 molar (M) stock solution of B2Pin2. Bis(1,5-cyclooctadiene)di-μ-methoxydiiridium(I) ([Ir(OMe)cod]2; 33.1 mg, 0.050 mmol) was weighed into a 20 mL vial and dissolved in THF (2 mL). The resulting solution was transferred to a 10 mL volumetric flask. The vial was washed with THF (3×1 mL), and the resulting solutions were transferred to the volumetric flask. The solution in the volumetric flask was diluted to the 10 mL mark with THF, giving a 0.0050 M stock solution of [Ir(OMe)cod]2. A ligand (0.10 mmol) was weighed into a 20 mL vial and dissolved in THF (2 mL). The resulting solution was transferred to a 10 mL volumetric flask. The vial was washed with THF (3×1 mL), and the resulting solutions were transferred to the volumetric flask. The solution in the volumetric flask was diluted to the 10 mL mark with THF, giving a 0.010 M stock solution of ligand. A J-Young NMR tube was charged with the 0.0050 M stock solution of [Ir(OMe)cod]2 (200 microliters (μL), 0.001 mmol), the 0.10 M stock solution of B2Pin2 (1.0 mL, 0.1 mmol), and the 0.010 M stock solution of ligand (200 μL, 0.002 mmol). The substrate (1.0 mmol) was added to the tube. The J-Young NMR tube was capped and shaken well to mix the liquids, removed from the glovebox and heated in an oil bath at 80° C. The reaction progress was monitored by removing the tube from the oil bath occasionally and acquiring 19F and 11B NMR spectra. After the reaction was judged complete, the volatiles were removed by rotary evaporation. The residue was then purified by Kugelrohr distillation to give the regiochemical mixture of borylated products. The ratio of steric to electronic products was determined by 19F NMR spectroscopy and GC-FID.0271] For 2-(3-chloro-4-ethoxy-5-fluorophenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (major): 1H NMR (500 MHz, acetone-d6) δ 7.52 (s, 1H), 7.37 (m, 1H), 4.22 (q, J=6.9 Hz, 2H), 1.38 (t, J=6.9 Hz, 3H, overlapping with the other isomer), 1.33 (s, 12H); 19F NMR (470 MHz, acetone-d6) δ -129.3 (d, J=10.0 Hz). [0272] For 2-(4-chloro-3-ethoxy-2-fluorophenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (minor): 1H NMR (500 MHz, acetone-d6) δ 7.37 (m, 1H), 7.24 (dd, J=8.3, 1.4 Hz, 1H), 4.14 (q, J=6.9 Hz, 2H), 1.38 (t, J=6.9 Hz, 3H), 1.34 (s, 12H); 19F NMR (470 MHz, acetone-d6) δ -117.9 (d, J=5.0 Hz). [0273] For the mixture: 13C NMR (125 MHz, acetone-d6) δ 161.5 (d, J=254.2 Hz), 156.8 (d, J=248.9 Hz), 146.7 (d, J=13.9 Hz), 144.3 (d, J=15.7 Hz), 132.8 (d, J=3.8 Hz), 132.4 (d, J=3.1 Hz), 131.6 (d, J=9.1 Hz), 126.1 (d, J=3.6 Hz), 121.7 (d, J=18.1 Hz), 85.3, 84.9, 70.9 (d, J=5.1 Hz), 70.8 (d, J=4.3 Hz), 25.2, 25.0, 15.9, 15.9. |
|
Stage #1: bis(pinacol)diborane With (1,5-cyclooctadiene)(methoxy)iridium(I) dimer; 4,4′,5,5′-Tetrahydro-2,2′-bioxazole for 0.5h; Inert atmosphere;
Stage #2: 1-chloro-2-ethoxy-3-fluorobenzene at 20℃; for 24h; Overall yield = 25.3 %; Overall yield = 152 mg; |
1 Example 1
Borylation of 1-chloro-2-ethoxy-3-fluorobenzene
General procedure: Example 1Borylation of 1-chloro-2-ethoxy-3-fluorobenzeneIn a nitrogen atmosphere glovebox, [Ir(OMe)cod]2 (33.1 mg, 0.05 mmol) was placed in a 20 mL vial. The solid was partially dissolved in N-methylpyrrolidin-2-one (NMP; 1 mL) and transferred to a 10 mL volumetric flask. The remaining residue in the vial was treated with NMP (4×1 mL), and the resulting solutions were transferred to the volumetric flask. The mixture was then diluted with NMP up to the 10 mL mark resulting in a 0.005 M [Ir(OMe)cod]2 stock solution. 4,4′,5,5′-Tetrahydro-2,2′-bioxazole (box; 14.0 mg, 0.1 mmol) was placed in a 20 mL vial. The solid was partially dissolved in NMP (1 mL) and transferred to a 10 mL volumetric flask. The remaining residue in the vial was treated with NMP (4×1 mL), and the resulting solutions were transferred to the volumetric flask. The mixture was diluted with NMP up to the 10 mL mark resulting in a 0.01 M box stock solution. [0285] To a 20 mL glass vial fitted with a micro stir bar and containing B2Pin2 (254 mg, 1.0 mmol) were added the [Ir(OMe)cod]2 stock solution (2.0 mL, 0.01 mmol [Ir(OMe)cod]2) and the box stock solution (2.0 mL, 0.02 mmol box). The mixture was stirred for 30 minutes (min). 1-Chloro-2-ethoxy-3-fluorobenzene (750 μL, 5 mmol) was added. The reaction was stirred for 24 hours (h) at room temperature (rt). Gas chromatograph-mass spectrometry (GC-MS) analysis showed full consumption of B2Pin2. GC-FID showed a 65:35 ratio of the electronic:steric product. The crude reaction mixture was stirred with water (20 mL) and extracted with diethyl ether (Et2O; 3×5 mL). The ethereal phase was dried over magnesium sulfate (MgSO4), filtered, and the solvent was removed under reduced pressure. The residue was purified by Kugelrohr distillation (2 mmHg). The first fraction (rt) was starting 1-chloro-2-ethoxy-3-fluorobenzene, isolated as a colorless oil (afforded 411 mg, 47% based on substrate). The second fraction (-100° C.) contained a mixture of 2-(4-chloro-3-ethoxy-2-fluorophenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane and 2-(3-chloro-4-ethoxy-5-fluorophenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (19F NMR ratio 66:34 electronic:steric product, GC-FID ratio 66:34 electronic:steric product), as a colorless oil (152 mg, 25.3% based on boron). [0286] For 2-(3-chloro-4-ethoxy-5-fluorophenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (minor): 1H NMR (500 MHz, acetone-d6) δ 7.52 (s, 1H), 7.37 (m, 1H, overlapping with the other isomer), 4.22 (q, J=6.9 Hz, 2H), 1.38 (t, J=6.9 Hz, 3H, overlapping with the other isomer), 1.33 (s, 12H, overlapping with the other isomer); 19F NMR (470 MHz, acetone-d6) δ -129.3 (d, J=10.0 Hz). [0287] For 2-(4-chloro-3-ethoxy-2-fluorophenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (major): 1H NMR (500 MHz, acetone-d6) δ 7.37 (m, 1H, overlapping with the other isomer), 7.24 (dd, J=8.3, 1.4 Hz, 1H), 4.14 (q, J=6.9 Hz, 2H), 1.38 (t, J=6.9 Hz, 3H, overlapping with the other isomer), 1.34 (s, 12H, overlapping with the other isomer); 19F NMR (470 MHz, acetone-d6) δ -117.9 (d, J=5.0 Hz). [0288] For the mixture: 13C NMR (125 MHz, acetone-d6) δ 161.5 (d, J=254.2 Hz), 156.8 (d, J=248.9 Hz), 146.7 (d, J=13.9 Hz), 144.3 (d, J=15.7 Hz), 132.8 (d, J=3.8 Hz), 132.4 (d, J=3.1 Hz), 131.6 (d, J=9.1 Hz), 126.1 (d, J=3.6 Hz), 121.7 (d, J=18.1 Hz), 85.3, 84.9, 70.9 (d, J=5.1 Hz), 70.8 (d, J=4.3 Hz), 25.2, 25.0, 15.9, 15.9. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping