| 81% |
With palladium diacetate; caesium carbonate; tricyclohexylphosphine In 1,4-dioxane at 90℃; for 24h; Inert atmosphere; |
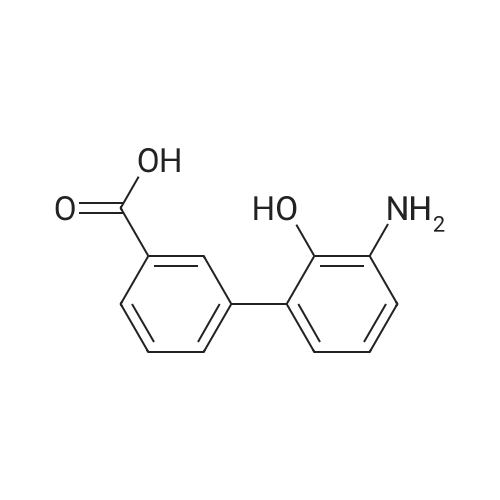
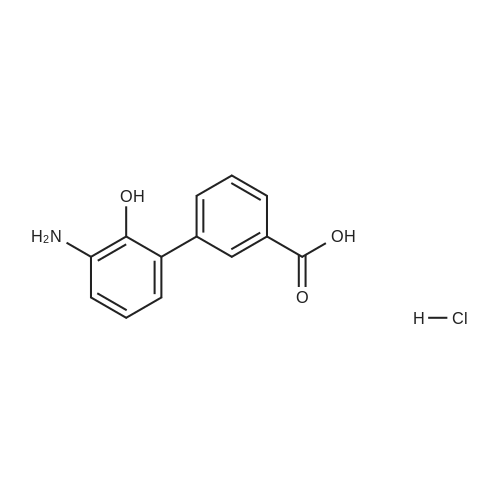
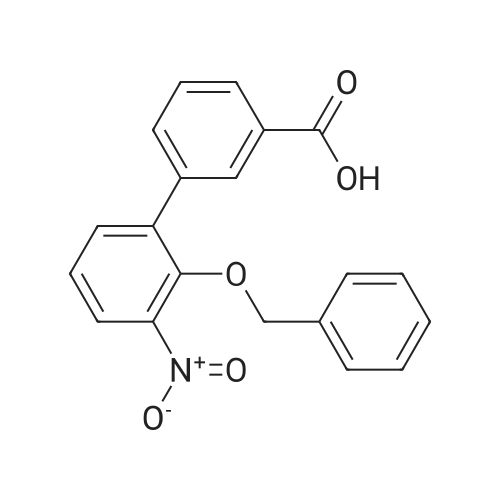
3 Example 3: Preparation of 2'-(benzyloxy)-3'-nitro-1 ,1 '-biphenyl-3-carboxylic acid
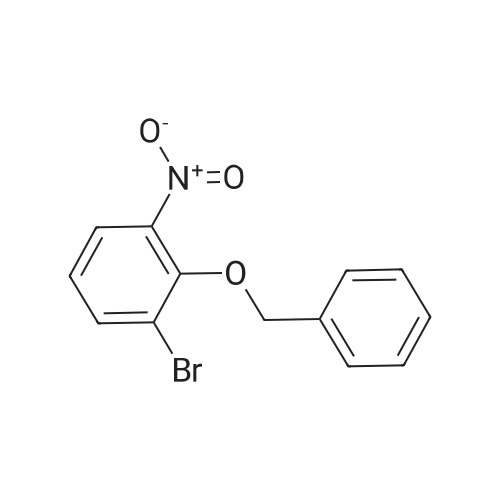
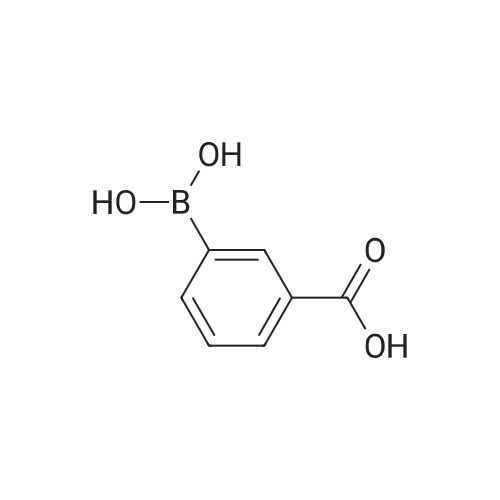
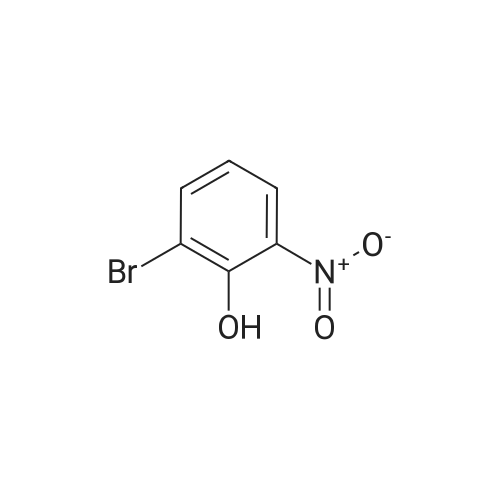
A mixture of 2-(benzyloxy)-1 -bromo-3-nitrobenzene (80 mg, 0.259 mmol), carboxyphenylboronic acid (45 mg, 0.27 mmol), Cs2CO3 (183 mg, 0.518 mmol), Tricyclohexylphosphine (0.014 g, 0.05 mmol) and 1 ,4-dioxane was desoxigenated with Ar and then Pd(OAc)2 (3 mg, 0.012 mmol) was added. The mixture was heated at 90 °C for 24 h and then cooled down to room temperature, filtered through a pad of Celite and the pad was washed with EtOAc. The filtrate was washed with water, HCI 15% and water, and was dried over sodium sulfate, filtered and concentrated to give a brown solid. The crude was purified by flash chromatography over silica (MeOH/CH2CI2; 6:94) to give 2'-(benzyloxy)-3'-nitro-1 ,1 '-biphenyl-3-carboxylic acid as a brown solid (83 mg, 81 %) |
| 80% |
With palladium diacetate; potassium carbonate; triphenylphosphine In tetrahydrofuran for 12h; Inert atmosphere; Reflux; |
2 Example 2: Synthesis of 2’-benzyloxy-3’-nitrobiphenyl-3-carboxylic acid of formula (III)
Triphenylphosphine (5.62 g, 21.4 mmol) is dissolved in THF (50 ml) in a 100 ml flask with magnetic stirrer, purged under nitrogen atmosphere, and palladium acetate (1.6 g, 7.14 mmol) is added. The suspension is maintained under stirring for one hour at room temperature, and then added to a suspension of benzyl 2-bromo-6-nitrophenyl ether (IV) (110 g, 0,357 mol), obtained as in example 1, and K2CO3 (128 g, 0.93 mol) in THF (660 ml), prepared in an inertised 1 L flask with mechanical stirrer, thermometer and condenser. The reaction mixture is then heated to reflux temperature and treated with 3-carboxyphenylboronic acid (65.2 g, 0.393 mol), added solid in portions. The reaction mixture is maintained at the same temperature for 12 hours, and then cooled to about 25°C, diluted with water (400 ml) and treated with concentrated H2SO4 (92 g, 0.94 mol). The solvent is then distilled at low pressure, and the product is extracted in dichloromethane (3 x 300 ml). The combined organic phases are washed with water, dried on sodium sulphate, filtered and concentrated at low pressure. The solid residue thus obtained is suspended in water (500 ml), and the suspension is heated at reflux temperature until completely dissolved. The solution is then cooled to about 0°C, and the solid product obtained is filtered through a Büchner funnel. The solid is washed with water and stove-dried at 50°C. 100 g of 2'-benzyloxy-3'-nitrobiphenyl-3-carboxylic acid of formula (III) is obtained, with a yield of 80% and HPLC purity exceeding 98%. [0058] The compound of formula (III) thus obtained presents an XRPD spectrum substantially as reported in Figure 1, wherein the most intense peaks, expressed in 2θ, are found at about 6.44; 8.42; 12.87; 15.57; 15.91; 18,35; 19.35; 20.25; 20.88; and 22.46 ± 0.2°. [0059] 1H-NMR (DMSO-d6, 300 MHz), δ: 8.12 (s, 1H); 8.05 (d, 1H); 7.92 (d, 1H); 7.82 (d, 1H), 7.76 (d 1H), 7.60 (t, 1H), 7.45 (t, 1H), 7.30-7.19 (m, 3H), 7.02-6.94 (m, 2H), 4.65 (s, 2H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping