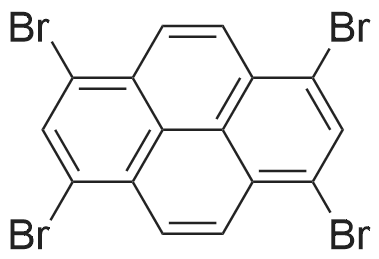
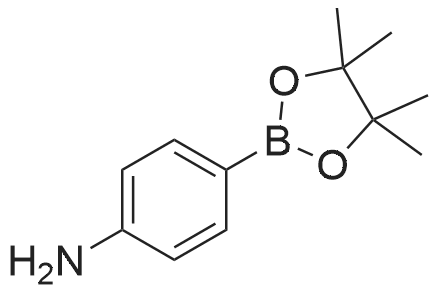
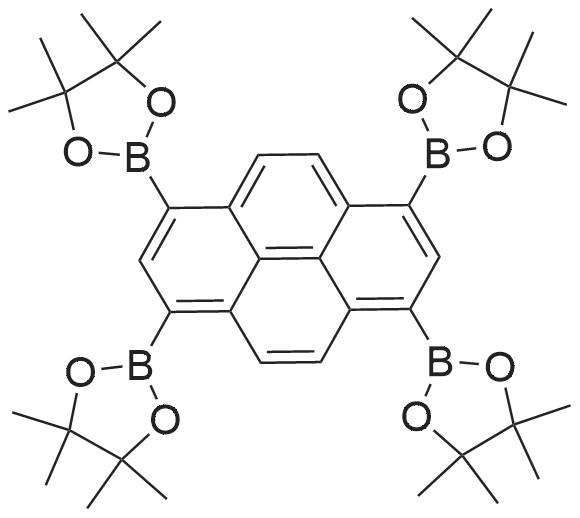
| 90% |
With tetrakis-(triphenylphosphine)-palladium; potassium carbonate In 1,4-dioxane; lithium hydroxide monohydrate at 115℃; for 72h; Inert atmosphere; Schlenk technique; |
|
| 90% |
With tetrakis-(triphenylphosphine)-palladium; potassium carbonate In 1,4-dioxane; lithium hydroxide monohydrate at 115℃; for 72h; Inert atmosphere; Schlenk technique; Glovebox; |
|
| 86% |
With tetrakis-(triphenylphosphine)-palladium; potassium carbonate In 1,4-dioxane; lithium hydroxide monohydrate at 110℃; for 72h; Inert atmosphere; |
|
| 86% |
With tetrakis-(triphenylphosphine)-palladium; potassium carbonate In 1,4-dioxane; lithium hydroxide monohydrate at 115℃; for 72h; |
|
| 84% |
With tetrakis-(triphenylphosphine)-palladium; potassium carbonate In 1,4-dioxane; lithium hydroxide monohydrate at 115℃; for 72h; Inert atmosphere; |
|
| 84% |
With tetrakis-(triphenylphosphine)-palladium; potassium carbonate In 1,4-dioxane; lithium hydroxide monohydrate at 115℃; for 72h; Inert atmosphere; |
|
| 81% |
With tetrakis-(triphenylphosphine)-palladium; potassium carbonate In 1,4-dioxane at 115℃; for 72h; Inert atmosphere; |
|
| 75% |
With tetrakis-(triphenylphosphine)-palladium; potassium carbonate In 1,4-dioxane for 72h; Reflux; Inert atmosphere; |
|
| 75% |
With tetrakis-(triphenylphosphine)-palladium; potassium carbonate In 1,4-dioxane; lithium hydroxide monohydrate at 115℃; for 72h; |
|
| 70% |
With tetrakis-(triphenylphosphine)-palladium; anhydrous potassium carbamate In 1,4-dioxane; lithium hydroxide monohydrate for 72h; Inert atmosphere; Reflux; |
|
| 64% |
With tetrakis-(triphenylphosphine)-palladium; potassium carbonate In 1,4-dioxane; lithium hydroxide monohydrate for 72h; Inert atmosphere; Reflux; |
|
| 61% |
With tetrakis-(triphenylphosphine)-palladium; potassium carbonate In 1,4-dioxane for 72h; Reflux; Inert atmosphere; |
|
| 58% |
With tetrakis-(triphenylphosphine)-palladium; potassium carbonate In 1,4-dioxane for 72h; Inert atmosphere; Reflux; |
|
| 54% |
With tetrakis-(triphenylphosphine)-palladium; potassium carbonate In 1,4-dioxane Inert atmosphere; Reflux; |
|
|
With tetrakis-(triphenylphosphine)-palladium; potassium carbonate In 1,4-dioxane; lithium hydroxide monohydrate |
|
|
With tetrakis-(triphenylphosphine)-palladium; potassium carbonate In 1,4-dioxane; lithium hydroxide monohydrate for 72h; Inert atmosphere; Reflux; |
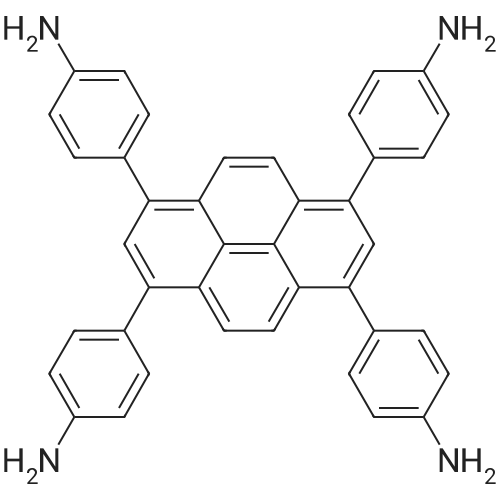
1,3,6,8-tetrakis(4-aminophenyl)pyrene (2) :
1,3,6,8- tetrabromopyrene (2.96 g, 5.72 mmol), 4- aminophenylboronic acid pinacol ester (6.0 g, 27.4 mmol), K2CO3 (4.4 g, 31.6 mmol), and Pd(PPh3)4 (0.66 g, 0.589 mmol) were introduced into a mixture of 1,4- dioxane (100 mL) and H2O (20 mL). The resulting mixture was refluxed under N2 atmosphere for 3 d. After cooling to room temperature, the solution was poured into water. The formed precipitate was filtered off, and washed with water and methanol, which was further purified by flash chromatography with acetone as eluent to afford the title compound as a yellow-brown solid. 1H NMR (400 MHz, d6-DMSO, 298K, TMS): d 8.13 (s, 4H), 7.79 (s, 2H), 7.35 (d, 8H, J=8.4 Hz), 6.77 (d, 8H, J=8.0 Hz), 5.32 (s, 8H) ppm. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping