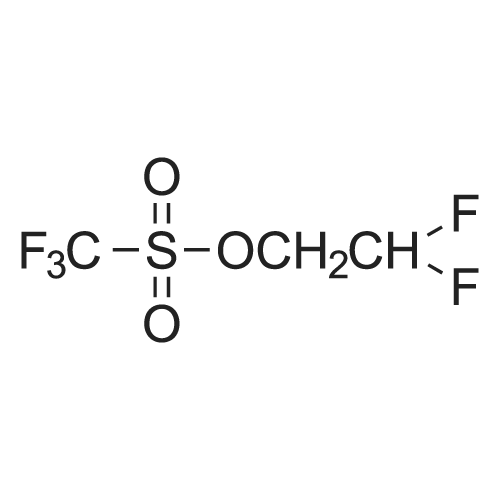
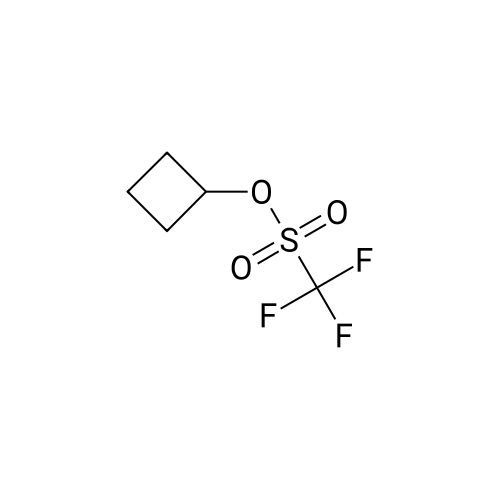
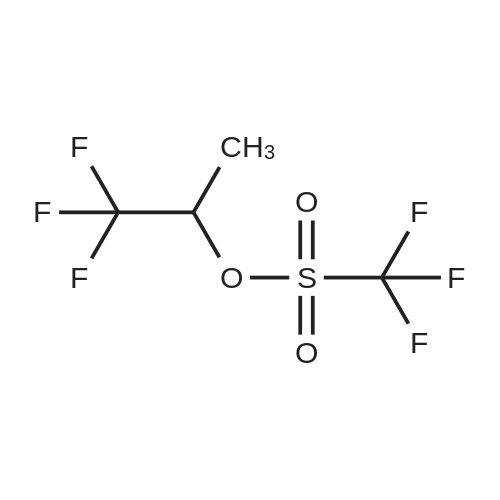
|
With pyridine; In dichloromethane; at 0 - 20℃; for 2h; |
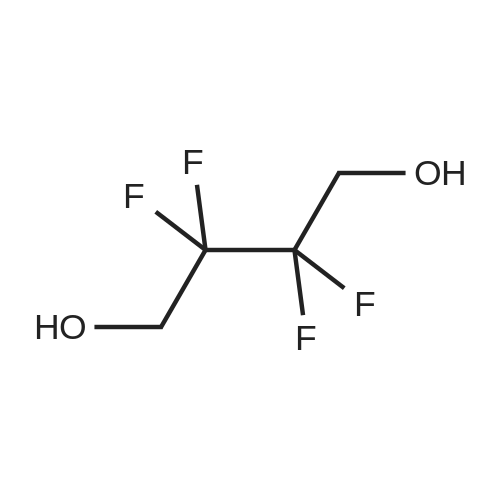
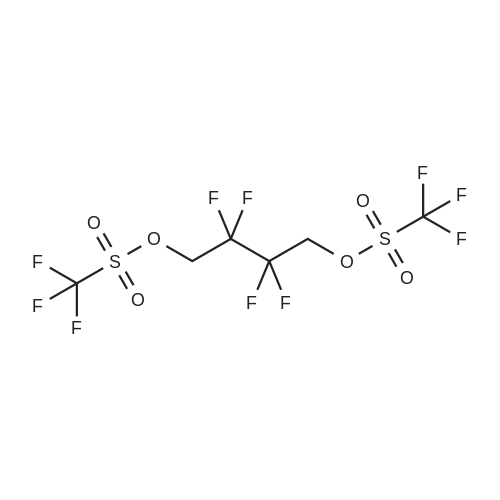
To a cooled (about 0 C.) solution of 2,2,3,3-tetrafluorobutanediol (15 grams, 93 mmol) and pyridine (19 mL, 230 mmol) in dichloromethane (250 mL), was added dropwise trifluoromethanesulfonic anhydride (34 mL, 200 mmol). After the addition, the mixture was stirred at about 0 C. for about one hour, followed by stirring at room temperature for one additional hour, then diluted with dichloromethane, washed with water and brine, dried over magnesium sulfate, filtered and concentrated to near dryness, leaving a dichloromethane-containing oil. 1H NMR (400 MHz, CDCl3) ? 4.82 (m). 19F NMR (376 MHz, CDCl3) ? -120.83 (m, 4H), -74.38 (s, 6H). |
|
With pyridine; In dichloromethane; at 0 - 20℃; for 2h; |
To a cooled (about 0 C.) solution of 2,2,3,3-tetrafluorobutanediol (15 grams, 93 mmol) and pyridine (19 mL, 230 mmol) in dichloromethane (250 mL), was added dropwise trifluoromethanesulfonic anhydride (34 mL, 200 mL). After the addition, the mixture was stirred at about 0 C. for about one hour, followed by stirring at room temperature for one additional hour, then diluted with dichloromethane, washed with water and brine, dried over magnesium sulfate, filtered and concentrated to near dryness, leaving a dichloromethane-containing oil. 1H NMR (400 MHz, CDCl3) delta 4.82 (m). 19F NMR (376 MHz, CDCl3) delta -120.83 (m, 4H), -74.38 (s, 6H). |
|
With pyridine; In diethyl ether; at 0 - 20℃; for 3.33333h; |
To an ice cold solution (00C internal temp) of 5 g of 2,2,3,3-tetrafluoro-l,4-butanediol and 9 mL of anhydrous pyridine (3.5 equiv) in 100 mL of anhydrous ether was added dropwise 15.4 mL of triflic anhydride (3 equiv) dropwise, keeping the internal temperature below 15C. After 20 min stirring in the ice bath, the mixture was allowed to warm to room temperature and stirred for 3 h. The mixture was then filtered, concentrated on the rotovap, redissolved in anhydrous ether, refiltered and concentrated again on the rotovap. Drying under vacuum gave an air stable, white crystalline solid: 1HnuMR (400 MHz,CDC13) 4.8 (complex symmetrical multiple.). |
|
With pyridine; In dichloromethane; at 0 - 20℃; for 1h; |
Step A: 2,2,3,3-Tetrafluorobutane-1,4-diol (0.207 g, 1.28 mmol) in DCM was cooled to 0 C. Pyridine (0.25 g, 3.19 mmol) was added, followed by addition of triflate anhydride (0.78 g, 2.75 mmol). The resulting mixture was then stirred at room temperature for an additional 1 h, and then diluted with DCM. The resulting mixture was washed with water, brine, then dried (Na2SO4) and concentrated to yield trifluoromethanesulfonic acid 2,2,3,3-tetrafluoro-4-trifluoromethanesulfonyloxy-butyl ester as white solid (0.45 g). The white solid (0.356 g, 0.835 mmol) in DMF (5 mL) was added dropwise to a mixture of 2-(1,3-dioxo-1,3-dihydro-isoindol-2-ylmethyl)-4,5-dihydroxy-benzonitrile (0.18 g, 0.62 mmol) and K2CO3 (0.17 g, 1.23 mmol) in DMF (5 mL) at 75 C. After addition, the resulting mixture was maintained at 75 C. for an additional 1 h. The resulting solid was filtered out and the filtrate evaporated to yield a residue. (0.2 g). MS MH+=421. |
|
With pyridine; at -5 - 20℃; for 1h; |
To a methylene chloride solution of 19.45 g (120 mmol; mmol in terms of pure content) of the crude product containing the compound (3) obtained in Example 1 and 23.7 g (300 mmol) of pyridine was dropwise added 72.8 g (257 mmol) of trifluoromethanesulfonic anhydride in the range of -5 to 5 C., which was then reacted at the same temperature for one hour and further at room temperature for 1 hour. The reaction solution was then washed with water and dried with magnesium sulfate, followed by distilling off the solvent to provide a crude product containing 2,2,3,3-tetrafluoro-1,4-bis(trifluoromethanesulfonyloxy)-butane, a desired product. |
|
|
General procedure: 2,2,3,3-Tetrafluoro-1,4-butanediol (1 mmol), 2,2,3,3,4,4,5,5-octafluoro-1,6-hexanediol, 1b (1 mmol), pyridine (2.5 mmol) and dichloromethylene (20 mL) were stirred at room temperature. After 20 min, trifluoromethanesulfonic anhydride (2.5 mmol) in 10 mL dichloromethylene was slowly added over 1 h. The mixture was stirred for 12 h, then washed with water (3 × 30 mL), and dried over anhydrous sodium sulfate. The solvent was removed under vacuum to give trifluoromethanesulfonic acid polyfluoroalkyldiyl ester [33], 1a-1b |
|
|
2,2,3,3-Tetrafluoro-1,4-butanediol 1a, or 1,4-butanediol 1b (1mmol), pyridine (3mmol) and dichloromethylene (20mL) were stirred at room temperature. After 30min, trifluoromethanesulfonic anhydride (2.5mmol) in 10mL dichloromethylene was slowly added over 1h. The mixture was stirred for 12h, then washed with water (3×30mL), and dried over anhydrous sodium sulfate. The solvent was removed under vacuum to give trifluoromethanesulfonic acid 2,2,3,3-tretrafluoro-1,4-butanedily ester. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +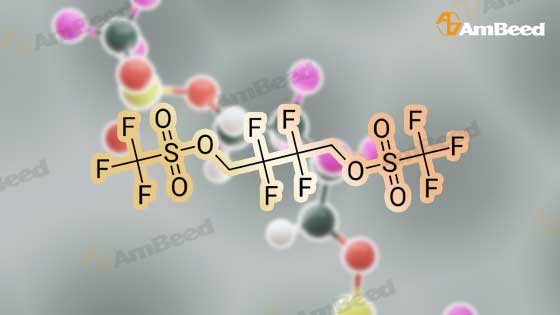

 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping